Seres Therapeutics, Inc. | |||||||||||
PROXY STATEMENT | |||||||||||
Annual Meeting of Stockholders
8:00 a.m. Eastern Time | |||||||||||
PRELIMINARY PROXY MATERIALS
SERES THERAPEUTICS, INC.
200 SIDNEY STREET101 CAMBRIDGEPARK DRIVE
CAMBRIDGE, MASSACHUSETTS 0213902140
April 29, 2022
[ ], 2024
To Our Stockholders:
You are cordially invited to attend the 20222024 Annual Meeting of Stockholders of Seres Therapeutics, Inc. at 8:00 a.m. Eastern time, on Wednesday, June 22, 2022Thursday, April 4, 2024 (the “Annual Meeting”). As part of our effort to maintain a safe and healthy environment for our directors, members of management and stockholders who wish to attend the Annual Meeting, theThe Annual Meeting will be held entirely online again this year.online. You will be able to attend the Annual Meeting online and submit your questions during the meeting by visiting www.virtualshareholdermeeting.com/MCRB2022.MCRB2024.
The Notice of Meeting and Proxy Statement on the following pages describe the matters to be presented at the Annual Meeting. Please see the section called “Who Can Attend the 20222024 Annual Meeting of Stockholders?” on page 43 of the proxy statement for more information about how to attend the meeting online.
Whether or not you attend the Annual Meeting webcast, it is important that your shares be represented and voted at the Annual Meeting. Therefore, I urge you to promptly vote and submit your proxy via the Internet or if you received paper copies of these materials, by signing, dating and returning the enclosed proxy card in the enclosed envelope, which requires no postage if mailed in the United States. If you have previously received our Notice of Internet Availability of Proxy Materials, then instructions regarding how you can vote are contained in that notice. If you have received a proxy card, then instructionsInstructions regarding how you can vote are contained on the proxy card. You may also vote online during the Annual Meeting. Instructions on how to vote during the meeting will be available at www.virtualshareholdermeeting.com/MCRB2022.MCRB2024.
Thank you for your support.
Sincerely,
Sincerely,

Eric D. Shaff
President and Chief Executive Officer
1 | ||||
1 | ||||
1 | ||||
2 | ||||
Questions and Answers About the | ||||
3 | ||||
7 | ||||
7 | ||||
Proposal 2: Ratification of Appointment of Independent Registered Public Accounting Firm | 11 | |||
12 | ||||
13 | ||||
15 | ||||
16 | ||||
19 | ||||
Independent Registered Public Accounting Firm Fees and Other Matters | ||||
20 | ||||
21 | ||||
22 | ||||
22 | ||||
22 | ||||
22 | ||||
22 | ||||
23 | ||||
23 | ||||
24 | ||||
25 | ||||
25 | ||||
25 | ||||
25 | ||||
26 | ||||
26 | ||||
27 | ||||
| 28 | |||
29 | ||||
29 | ||||
| 29 | |||
35 | ||||
36 | ||||
38 | ||||
41 | ||||
41 | ||||
43 | ||||
Security Ownership of Certain Beneficial Owners and Management | ||||
44 | ||||
46 | ||||
Compensation and Talent Committee Interlocks and Insider Participation | ||||
49 | ||||
50 | ||||
51 | ||||
52 | ||||
53 | ||||
Notice of Annual Meeting of Stockholders
To Be Held Wednesday, June 22, 2022Thursday, April 4, 2024
SERES THERAPEUTICS, INC.
200 SIDNEY STREET101 CAMBRIDGEPARK DRIVE
CAMBRIDGE, MASSACHUSETTS 0213902140
The Annual Meeting of Stockholders (the “Annual Meeting”) of Seres Therapeutics, Inc., a Delaware corporation (the “Company”), will be held at 8:00 a.m. Eastern time on Wednesday, June 22, 2022.Thursday, April 4, 2024. The Annual Meeting will be a completely virtual meeting, which will be conducted via live webcast. You will be able to attend the Annual Meeting online and submit your questions during the meeting by visiting www.virtualshareholdermeeting.com/MCRB2022MCRB2024 and entering your 16-digit control number included on your Notice of Internet Availability of Proxy Materials (“Internet Notice”), your proxy card or on the instructions that accompanied your proxy materials. The Annual Meeting will be held for the following purposes:
|
|
|
|
Holders of record of our common stock (our “Common Stock”)Common Stock as of the close of business on April 25, 2022February 12, 2024 are entitled to notice of and to vote at the Annual Meeting, or any continuation, postponement or adjournment of the Annual Meeting. To participate in the Annual Meeting, including to vote via the Internet, you will need the 16-digit control number included on your Internet Notice, your proxy card or on the instructions that accompanied your proxy materials. A complete list of stockholders entitled to vote at the Annual Meeting will be open to the examination of any stockholder for a purpose germane to the meeting by sending an email to info@serestherapeutics.com, stating the purpose of the request and providing proof of ownership of our Common Stock for a period of ten days prior toending on the day before the Annual Meeting and will also be available on the bottom of your screen during the Annual Meeting after entering your 16-digit control number.Meeting. The Annual Meeting may be continued or adjourned from time to time without notice other than by announcement at the Annual Meeting.
It is important that your shares be represented regardless of the number of shares you may hold. Whether or not you plan to attend the Annual Meeting webcast, we urge you to vote your shares via the Internet, as described in the enclosed materials. If you have received a printed copy of theyour proxy card by mail, you may alternatively sign, date and mail the proxy card in the enclosed return envelope. Promptly voting your shares will ensure the presence of a quorum at the Annual Meeting and will save us the expense of further solicitation. Submitting your proxy now will not prevent you from voting your shares at the Annual Meeting if you desire to do so, as your proxy is revocable at your option.
By Order of the Board of Directors

Thomas J. DesRosier
Secretary
Cambridge, Massachusetts
April 29, 2022[ ], 2024
SERES THERAPEUTICS, INC.
200 SIDNEY STREET101 CAMBRIDGEPARK DRIVE
CAMBRIDGE, MASSACHUSETTS 0213902140
This proxy statement is furnished in connection with the solicitation by the Board of Directors of Seres Therapeutics, Inc. of proxies to be voted at our Annual Meeting of Stockholders to be held on Wednesday, June 22, 2022Thursday, April 4, 2024 (the “Annual Meeting”), at 8:00 a.m. Eastern time, and at any continuation, postponement, or adjournment of the Annual Meeting. The Annual Meeting will be a completely virtual meeting, which will be conducted via live webcast. You will be able to attend the Annual Meeting online and submit your questions during the meeting by visiting www.virtualshareholdermeeting.com/ MCRB2022MCRB2024 and entering your the 16-digit control number included on your Notice of Internet Availability of Proxy Materials (the “Internet Notice”), on your proxy card or on the instructions that accompanied your proxy materials. Holders of record of shares of our common stock (our “Common Stock”), as of the close of business on April 25, 2022February 12, 2024 (the “Record Date”), will be entitled to notice of and to vote at the Annual Meeting and any continuation, postponement, or adjournment of the Annual Meeting. As of the Record Date, there were 92,230,428 145,557,567
shares of Common Stock outstanding and entitled to vote at the Annual Meeting. Each share of Common Stock is entitled to one vote on any matter presented to stockholders at the Annual Meeting.
This proxy statement and our Annual Report to Stockholders for the year ended December 31, 20212023 (the “2021“2023 Annual Report”) will be released on or about April 29, 2022March 5, 2024 to our stockholders on the Record Date.
In this proxy statement, “Seres”, “Company”, “we”, “us”, and “our” refer to Seres Therapeutics, Inc.
IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE STOCKHOLDER MEETING TO BE HELD ON WEDNESDAY, JUNE 22, 2022THURSDAY, APRIL 4, 2024
This Proxy Statement and our 20212023 Annual Report to Stockholders are available at http://www.proxyvote.com/.
PROPOSALS
At the Annual Meeting, our stockholders will be asked:
•
•
•
•
Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the Notice of Annual Meeting or brought before the meeting by or at the direction of our Board of Directors or by a stockholder of record on the Record Date for the meeting who is entitled to vote at the meeting and who has delivered timely written notice in proper form to our Corporate Secretary of the stockholder’s intention to bring such business before the meeting. As of the date of this proxy statement, we know of no other business that will be presented at the Annual Meeting. If any other
matter properly comes before the stockholders for a vote at the Annual Meeting, however, the proxy holders named on the Company’s proxy card will vote your shares in accordance with their best judgment.
RECOMMENDATIONS OF THE BOARD
Our Board of Directors (the “Board of Directors” or the “Board”) recommends that you vote your shares of Common Stock as indicated below. If you return a properly completed proxy card, or vote your shares by Internet, your shares will be
1
voted on your behalf as you direct. If not otherwise specified, shares of Common Stock represented by proxies will be voted, and the Board of Directors recommends that you vote:
•
•
•
If any other matter properly comes before the stockholders for a vote at the Annual Meeting, the proxy holders named on the Company’s proxy card will vote your shares in accordance with their best judgment.
INFORMATION ABOUT THIS PROXY STATEMENT
Why you Received this Proxy Statement. You are viewing or have received these proxy materials because our Board is soliciting your proxy to vote your shares of Common Stock at the Annual Meeting. This proxy statement includes information that we are required to provide to you under the rules of the Securities and Exchange Commission (“SEC”) and that is designed to assist you in voting your shares.
Notice of Internet Availability of Proxy Materials. As permitted by SEC rules, we are making this proxy statement and our 2021 Annual Report available to our stockholders electronically via the Internet. On or about April 29, 2022, we commenced mailing to our stockholders an Internet Notice containing instructions on how to access this proxy statement and our 2021 Annual Report and vote online. If you received an Internet Notice by mail, you will not receive a printed copy of the proxy materials unless you specifically request them. Instead, the Internet Notice instructs you on how to access and review all of the important information contained in the proxy statement and 2021 Annual Report. The Internet Notice also instructs you on how you may submit your proxy over the Internet. If you received an Internet Notice by mail and would like to receive a printed copy of our proxy materials, you should follow the instructions for requesting such materials contained on the Internet Notice.
Printed Copies of Our Proxy Materials. If you received printed copies of our proxy materials, then instructions Instructions regarding how you can vote are contained on the proxy card included in the proxy materials.
Householding. The SEC’s rules permit us and banks, brokers, or other agents to deliver a single set of proxy materials to one address shared by two or more of our stockholders. This delivery method is referred to as “householding” and can result in significant cost savings. To take advantage of this opportunity, we and certain banks, brokers, or other agents have delivered only one set of proxy materials to multiple stockholders who share an address, unless we received contrary instructions from the impacted stockholders prior to the mailing date. We agree to deliver promptly, upon written or oral request, a separate copy of the proxy materials, as requested, to any stockholder at the shared address to which a single copy of those documents was delivered. If you prefer to receive separate copies of the proxy materials, contact Broadridge Financial Solutions, Inc. at 1-866-540-7095 or in writing at Broadridge, Householding Department, 51 Mercedes Way, Edgewood, New York 11717.
If you are currently a stockholder sharing an address with another stockholder and wish to receive only one copy of future proxy materials for your household, please contact Broadridge at the above phone number or address.
2
Questions and Answers about the 20222024 Annual Meeting of Stockholders
WHO IS ENTITLED TO VOTE AT THE ANNUAL MEETING?Who is entitled to vote at the Annual Meeting?
The Record Date for the Annual Meeting is April 25, 2022.February 12, 2024. You are entitled to vote at the Annual Meeting only if you were a stockholder of record at the close of business on that date, or if you hold a valid proxy for the Annual Meeting. Each outstanding share of Common Stock is entitled to one vote for all matters before the Annual Meeting. At the close of business on the Record Date, there were 92,230,428145,557,567 shares of Common Stock outstanding and entitled to vote at the Annual Meeting.
WHAT IS THE DIFFERENCE BETWEEN BEING A “RECORD HOLDER” AND HOLDING SHARES IN “STREET NAME”?
What is the difference between being a "Record Holder" and holding shares in "Street Name"?
A record holder holds shares in his or her name. Shares held in “street name” means shares that are held in the name of a bank or broker on a person’s behalf.
AM
Am I ENTITLED TO VOTE IF MY SHARES ARE HELD IN “STREET NAME”entitled to vote if my shares are held in "Street Name"?
Yes. If your shares are held by a bank or a brokerage firm, you are considered the “beneficial owner” of those shares held in “street name.” If your shares are held in street name, these proxy materials are being provided to you by your bank or brokerage firm, along with a voting instruction card if you received printed copies of our proxy materials. As the beneficial owner, you have the right to direct your bank or brokerage firm how to vote your shares, and the bank or brokerage firm is required to vote your shares in accordance with your instructions. If your shares are held in “street name” and you would like to vote your shares online at the Annual Meeting, you should contact your bank or broker to obtain your 16-digit control number or otherwise vote through the bank or broker.
HOW MANY SHARES MUST BE PRESENT TO HOLD THE ANNUAL MEETING?
How many shares must be present to hold the Annual Meeting?
A quorum must be present at the Annual Meeting for any business to be conducted. The presence at the Annual Meeting, in person, or by remote communication, or represented by proxy, of the holders of a majority in voting power of the Common Stock issued and outstanding and entitled to vote on the Record Date will constitute a quorum.
WHO CAN ATTEND THE 2022 ANNUAL MEETING OF STOCKHOLDERS?
Who can attend the 2024 Annual Meeting of Stockholders?
You may attend the Annual Meeting online only if you are a Seres stockholder who is entitled to vote at the Annual Meeting, or if you hold a valid proxy for the Annual Meeting. You may attend and participate in the Annual Meeting by visiting the following website: www.virtualshareholdermeeting.com/ MCRB2022.MCRB2024.
The meeting webcast will begin promptly at 8:00 a.m. Eastern Time. We encourage you to access the meeting prior to the start time. Online check-in will begin at 7:55 a.m. Eastern Time, and you should allow amplesufficient time for the check-in procedures.
To attend and participate in the Annual Meeting, you will need the 16-digit control number included on your Internet Notice, on your proxy card or on the instructions that accompanied your proxy materials. If your bank or broker holds your shares in street name, you should contact your bank or broker to obtain your 16-digit control number or otherwise vote through the bank or broker. If you lose your 16-digit control number, you may join the Annual Meeting as a “Guest” but you will not be able to vote or ask questionsquestions.
What if during the check-in time or accessduring the list of stockholders as ofAnnual Meeting I have technical difficulties or trouble accessing the Record Date.virtual meeting website?
WHAT IF DURING THE CHECK-IN TIME OR DURING THE ANNUAL MEETING I HAVE TECHNICAL DIFFICULTIES OR TROUBLE ACCESSING THE VIRTUAL MEETING WEBSITE?
We will have technicians ready to assist you with any technical difficulties you may have accessing the virtual meeting website. If you encounter any difficulties accessing the virtual meeting website during the check-in or meeting time, please call the technical support number that will be posted on the Annual Meeting login page.
WHY HOLD A VIRTUAL MEETING?
Why hold a virtual meeting?
As part of our effort to maintain a safe and healthy environment for our directors, members of management and stockholders who wish to attend the Annual Meeting, weWe believe that hosting a virtual meeting is in the best interest of the Company and its stockholders and enables increased stockholder attendance and participation because stockholders can participate from any location around the world. Stockholders will have the same rights and opportunities to participate as they would have at an in-person meeting.
WHAT IF A QUORUM IS NOT PRESENT AT THE ANNUAL MEETING?
What if a quorum is not present at the Annual Meeting?
If a quorum is not present at the scheduled time of the Annual Meeting, (i) the Chairperson of the Annual Meeting, or a person designated by the Chairperson of the Annual Meeting or (ii) a majority in voting power of the stockholders entitled
3
to vote thereon, present in person, or by remote communication, if applicable, or represented by proxy, may adjourn the Annual Meeting until a quorum is present or represented.
WHAT DOES IT MEAN IF
What does it mean if I RECEIVE MORE THAN ONE INTERNET NOTICE OR MORE THAN ONE SET OF PROXY MATERIALS?receive more than one set of proxy materials?
It means that your shares are held in more than one account at the transfer agent and/or with banks or brokers. Please vote all of your shares. To ensure that all of your shares are voted, for each Internet Notice or set of proxy materials, please submit your proxy via the Internet, or, if you received printed copies of the proxy materials, by signing, dating and returning theeach enclosed proxy card in the enclosed envelope.
HOW DO
How do I VOTE?vote?
Whether or not you expect to attend the Annual Meeting online, we urge you to vote your shares as promptly as possible to ensure your representation and the presence of a quorum at the Annual Meeting. If you submit your proxy, you may still decide to attend the Annual Meeting and vote your shares electronically. Your most recent proxy card or Internet proxy is the one that is counted.
Stockholders of Record. Record. If you are a stockholder of record, you may vote:
•
•
•
Internet voting facilities for stockholders of record will be available 24 hours a day and will close at 11:59 p.m. Eastern Time on June 21, 2022April 3, 2024 (other than the voting that occurs during the Annual Meeting). To participate in the Annual Meeting, including to vote via the Internet, you will need the 16-digit control number included on your Internet Notice, on your proxy card or on the instructions that accompanied your proxy materials.
Beneficial Owners of Shares Held in “Street Name.”If your shares are held in street name through a bank or broker, you will receive instructions on how to vote from the bank or broker. You must follow their instructions in order for your shares to be voted. Internet voting also may be offered to stockholders owning shares through certain banks and brokers. If your shares are not registered in your own name and you would like to vote your shares online at the Annual Meeting, you should contact your bank or broker to obtain your 16-digit control number or otherwise vote through the bank or broker.
If you lose your 16-digit control number, you may join the Annual Meeting as a “Guest” but you will not be able to vote or ask questions or access the list of stockholders as of the Record Date.questions. You will need to obtain your own Internet access if you choose to attend the Annual Meeting online and/or vote over the Internet.
CAN
Can I CHANGE MY VOTE AFTERchange my vote after I SUBMIT MY PROXY?submit my proxy?
Yes.
Yes.
If you are a registered stockholder, you may revoke your proxy and change your vote:
•
•
•
•
Your most recent proxy card or Internet proxy is the one that is counted. Your attendance at the Annual Meeting by itself will not revoke your proxy unless you give written notice of revocation to the Secretary before your proxy is voted or you vote online during the Annual Meeting.
If your shares are held in street name, you may change or revoke your voting instructions by following the specific directions provided to you by your bank or broker, or you may vote online during the Annual Meeting by obtaining your 16-digit control number or otherwise voting through the bank or broker.
WILL THERE BE A QUESTION AND ANSWER SESSION DURING THE ANNUAL MEETING?
4
Will there be a question and answer session during the Annual Meeting?
As part of the Annual Meeting, we will hold a live Q&A session, during which we intend to answer appropriate questions submitted by stockholders during the meeting that are pertinent to the Company and the meeting matters. The Company will endeavor to answer as many questions submitted by stockholders as time permits. Only stockholders that have accessed the Annual Meeting as a stockholder (rather than a “Guest”) by following the procedures outlined above in “Who Can Attend the 20222024 Annual Meeting of Stockholders?” will be permitted to submit questions during the Annual Meeting. Each stockholder is limited to no more than two questions. Questions should be succinct and only cover a single topic. We will not address questions that are, among other things:
•
•
•
•
•
•
•
•
•
Additional information regarding the Q&A session will be available in the “Rules of Conduct” available on the Annual Meeting webpage for stockholders that have accessed the Annual Meeting as a
stockholder (rather than a “Guest”) by following the procedures outlined above in “Who Can Attend the 20222024 Annual Meeting of Stockholders?”.
WHO WILL COUNT THE VOTES?
Who will count the votes?
A representative of Broadridge Financial Solutions, Inc., our inspector of election, will tabulate and certify the votes.
WHAT IF
What if I DO NOT SPECIFY HOW MY SHARES ARE TO BE VOTED?do not specify how my shares are to be voted?
If you submit a proxy but do not indicate any voting instructions, the persons named as proxies will vote in accordance with the recommendations of our Board of Directors. Our Board of Directors’ recommendations are indicated on page 21 of this proxy statement, as well as with the description of each proposal in this proxy statement.
WILL ANY OTHER BUSINESS BE CONDUCTED AT THE ANNUAL MEETING?
Will any other business be conducted at the Annual Meeting?
We know of no other business that will be presented at the Annual Meeting. If any other matter properly comes before the stockholders for a vote at the Annual Meeting, however, the proxy holders named on the Company’s proxy card will vote your shares in accordance with their best judgment.
HOW MANY VOTES ARE REQUIRED FOR THE APPROVAL OF THE PROPOSALS TO BE VOTED UPON AND HOW WILL ABSTENTIONS AND BROKER NON-VOTES BE TREATED?
How many votes are required for the approval of the proposals to be voted upon and how will abstentions and broker non-votes be treated?
Proposal | Votes required | Effect of Votes Withheld / Abstentions and Broker Non-Votes | ||
Proposal 1: Election of Directors | The plurality of the votes cast. This means that the | Votes withheld and broker non-votes will have no effect. | ||
Proposal 2: Ratification of Appointment of Independent Registered Public Accounting Firm | The affirmative vote of the holders of a majority in voting power of the votes cast affirmatively or negatively. | Abstentions will have no effect. We do not expect any broker non-votes on this proposal. | ||
Proposal 3: Approval, on an Advisory (Non- Binding) Basis, of the Compensation of Our Named Executive Officers | The affirmative vote of the holders of a majority in voting power of the votes cast affirmatively or negatively. | Abstentions and broker non-votes will have no effect. | ||
Proposal 4: Approval of an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of Common Stock | The affirmative vote of the holders of a majority of the stock of the Company entitled to vote at the Annual Meeting. | Abstentions will have the same effect as votes against. We do not expect any broker non-votes on this proposal. | ||
Proposal 5: Approval of an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4 | The affirmative vote of the holders of a majority of voting power of the votes cast affirmatively or negatively. | Abstentions will have no effect. We do not expect any broker non-votes on this proposal. | ||
Proposal 6: Vote upon a stockholder proposal on simple majority vote | The affirmative vote of the holders of a majority in voting power of the votes cast affirmatively or negatively. | Abstentions and broker non-votes will have no effect. |
WHAT IS AN ABSTENTION AND HOW WILL VOTES WITHHELD AND ABSTENTIONS BE TREATED?
5
What is an abstention and how will votes withheld and abstentions be treated?
A “vote withheld,” in the case of the proposal regarding the election of directors, or an “abstention,” with respect to the other proposals, represents a stockholder’s affirmative choice to decline to vote on a proposal. Votes withheld and abstentions are counted as present and entitled to vote for purposes of determining a quorum. Votes withheld have no effect on the election of directors (Proposal 1), abstentions will have the same effect as a vote against the approval of an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of Common Stock (Proposal 4), and abstentions have no effect on the other proposals.proposals (Proposals 2, 3, 5 and 6).
WHAT ARE BROKER NON-VOTES AND DO THEY COUNT FOR DETERMINING A QUORUM?
What are broker non-votes and do they count for determining a quorum?
Generally, broker non-votes occur when shares held by a broker in “street name” for a beneficial owner are not voted with respect to a particular proposal because the broker (1) has not received voting instructions from the beneficial owner and (2) lacks discretionary voting power to vote those shares. A broker is entitled to vote shares held for a beneficial owner on routine matters, such as the ratification of the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm (Proposal 2), the approval of an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of Common Stock (Proposal 4), and the approval of an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4 (Proposal 5) without instructions from the beneficial owner of those shares. On the other hand, absent instructions from the beneficial owner of such shares, a broker is not entitled to vote shares held for a beneficial owner on non-routine matters, such as the election of directors and(Proposal 1), the approval on an advisory (non-binding) basis of the compensation of our named executive officers.officers (Proposal 3), and the stockholder proposal on simple majority vote (Proposal 6). Those items for which your broker cannot vote result in broker non-votes if you do not provide your broker with voting instructions on such items. Broker non-votes count for purposes of determining whether a quorum is present.
WHERE CANWhere can I FIND THE VOTING RESULTS OF THE 2022 ANNUAL MEETING OF STOCKHOLDERS?find the voting results of the 2024 Annual Meeting of Stockholders?
We plan to announce preliminary voting results at the Annual Meeting and we will report the final results in a Current Report on Form 8-K, which we intend to file with the SEC after the Annual Meeting.
6
Election of Directors
At the Annual Meeting, three (3)two (2) Class IIII Directors are to be elected to hold office until the Annual Meeting of Stockholders to be held in 20252027 and until such director’s successor is elected and qualified.
We currently have nine (9)eight (8) directors on our Board, including three (3)two (2) current Class IIII Directors. Our current Class IIII Directors are Dennis A. Ausiello, M.D.,Paul R. Biondi, who has served on our Board since April 2015, Willard H. Dere, M.D.,March 2020, and Kurt C. Graves, who has served on our Board since July 2017, and Eric D. Shaff, who has served on our Board since January 2019.November 2015. The Board has nominated each of Dennis A. Ausiello, M.D., Willard H. Dere, M.D.,Paul R. Biondi and Eric D. ShaffKurt C. Graves for election as Class IIII Directors at the Annual Meeting.
The proposal regarding the election of directors requires the approval of a plurality of the votes cast. This means that the threetwo nominees receiving the highest number of affirmative “FOR” votes will be elected as Class IIII Directors. Votes withheld and broker non-votes are not considered to be votes cast and, accordingly, will have no effect on the outcome of the vote on this proposal.
As set forth in our Restated Certificate of Incorporation, the Board of Directors is currently divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. The members of the classes are divided as follows:
•
•
•
Our Restated Certificate of Incorporation and Amended and Restated Bylaws provide that the authorized number of directors may be changed only by resolution of our Board of Directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our Board of Directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of our Company. Our directors may be removed only for cause by the affirmative vote of the holders of at least two-thirds of our outstanding voting stock entitled to vote in the election of directors. There are no family relationships among any of our executive officers or directors.
If you submit a proxy but do not indicate any voting instructions, the persons named as proxies will vote the shares of Common Stock represented thereby for the election as Class IIII Directors of the persons whose names and biographies appear below. All of the persons whose names and biographies appear below are currently serving as our directors. In the event any of the nominees should become unable to serve, or for good cause will not serve, as a director, it is intended that votes will be cast for a substitute nominee designated by our Board of Directors or our Board of Directors may elect to reduce its size. The Board of Directors has no reason to believe that the nominees named below will be unable to serve if elected. Each of the nominees has consented to being named in this proxy statement and to serve if elected.
VOTE REQUIRED
The proposal regarding the election of directors requires the approval of a plurality of the votes cast. This means that the threetwo nominees receiving the highest number of affirmative “FOR” votes will be elected as Class IIII Directors. Votes withheld and broker non-votes are not considered to be votes cast and, accordingly, will have no effect on the outcome of the vote on this proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “FOR” the election of the below Class III Director nominees.
|
7
DIRECTOR NOMINEES AND CONTINUING DIRECTORS
Name | Age | Served as a Director Since | Position with Seres | Class and Year Term Ending |
Director Nominees | ||||
Paul R. Biondi | 54 | 2020 | Director | Class III (Subsequent Term Ending 2027 if elected at the Annual Meeting) |
Kurt C. Graves | 56 | 2015 | Director | Class III (Subsequent Term Ending 2027 if elected at the Annual Meeting) |
Continuing Directors | ||||
Dennis A. Ausiello, M.D. | 78 | 2015 | Director | Class I (Term Ending 2025) |
Stephen A. Berenson | 63 | 2019 | Chairman of the Board of Directors | Class II (Term Ending 2026) |
Willard H. Dere, M.D. | 70 | 2017 | Director | Class I (Term Ending 2025) |
Claire M. Fraser, Ph.D. | 68 | 2023 | Director | Class II (Term Ending 2026) |
Richard N. Kender | 68 | 2014 | Director | Class II (Term Ending 2026) |
Eric D. Shaff | 48 | 2019 | President, Chief Executive | Class I (Term Ending 2025) |
Name | Age | Served as a Director Since | Position with Seres | Class and Year Term Ending | ||||
Director Nominees | ||||||||
Dennis A. Ausiello, M.D. | 76 | 2015 | Director | Class I (Term Ending 2025 if elected at the Annual Meeting) | ||||
Willard H. Dere, M.D. | 68 | 2017 | Director | Class I (Term Ending 2025 if elected at the Annual Meeting) | ||||
Eric D. Shaff | 46 | 2019 | President, Chief Executive Officer and Director | Class I (Term Ending 2025 if elected at the Annual Meeting) | ||||
Continuing Directors | ||||||||
Stephen A. Berenson | 61 | 2019 | Chairman of the Board of Directors | Class II (Term Ending 2023) | ||||
Richard N. Kender | 66 | 2014 | Director | Class II (Term Ending 2023) | ||||
Meryl S. Zausner | 65 | 2018 | Director | Class II (Term Ending 2023) | ||||
Grégory Behar | 52 | 2014 | Director | Class III (Term Ending 2024) | ||||
Paul R. Biondi | 52 | 2020 | Director | Class III (Term Ending 2024) | ||||
Kurt C. Graves | 54 | 2015 | Director | Class III (Term Ending 2024) |
The principal occupations and business experience, for at least the past five years, of each Director (including the Class IIII Director nominees) are as follows:
PAUL R. BIONDI | Age 54 |
Paul R. Biondi has served as a member of our Board of Directors since March 2020. Mr. Biondi is an Executive Partner and President of Pioneering Medicines at Flagship Pioneering, a life sciences innovation firm which conceives, creates, resources and develops first-in-category bioplatform companies, roles he has held since November 2019. Mr. Biondi joined Flagship Pioneering following a seventeen-year tenure at Bristol-Myers Squibb, or BMS, a pharmaceutical company, where he was most recently the Senior Vice President of Strategy and Business Development from October 2015 to November 2019. Prior to serving in the role of Senior Vice President of Strategy, from 2002 to 2015, Mr. Biondi held a series of other leadership roles within BMS’ Research and Development organization overseeing strategy, portfolio, and project management, as well as clinical and business operations. Mr. Biondi holds a bachelor’s degree from Dartmouth College and an M.B.A. from the J.L. Kellogg School of Management at Northwestern University. We believe that Mr. Biondi is qualified to serve on our Board of Directors because of his extensive experience in biopharmaceutical strategy and corporate development.
KURT C. GRAVES | Age 56 |
Kurt C. Graves has served as a member of our Board of Directors since November 2015. Mr. Graves has served as the Chairman, President and Chief Executive Officer of i20 Therapeutics, Inc., a biotechnology company, since August 2023, and he previously served as the Executive Chairman of its board of directors from August 2021 to August 2023. Mr. Graves was previously the Chairman, President and Chief Executive Officer of Intarcia Therapeutics, Inc., a biotechnology company, from September 2010 to December 2020, and on its board of directors from August 2010 to December 2020. Previously, he served as Executive Vice President, Chief Commercial Officer and Head of Strategic Development at Vertex Pharmaceuticals Inc., or Vertex, from July 2007 to October 2009. Prior to joining Vertex, Mr. Graves held various senior leadership positions at Novartis Pharmaceuticals Corporation, or Novartis Corp., from 1999 to June 2007, including the Global General Medicines Business Unit Head and Global Chief Marketing Officer for the pharmaceuticals division of Novartis Corp. from September 2003 to June 2007. Prior to Novartis Corp., Mr. Graves held senior leadership positions at Merck and Astra-Merck where he led the U.S. Business Unit responsible for Prilosec, Nexium and Prilosec OTC over a 10-year period. He served as Chairman on the board of directors of Radius Health, Inc. from May 2011 to March 2020, and as a director on Achillion Pharmaceuticals, Inc., or Achillion, from June 2012 to January 2020, when Achillion was acquired. Mr. Graves received a B.S. in Biology from Hillsdale College. We believe Mr. Graves is qualified to serve as a member of our Board of Directors because of his extensive experience in the life sciences industry, membership on various boards of directors and his leadership and management experience.
DENNIS A. AUSIELLO, M.D. | Age |
Dennis A. Ausiello, M.D., has served as a member of our Board of Directors since April 2015. Dr. Ausiello has served as the Jackson Distinguished Professor of Clinical Medicine at Harvard Medical School and Director, Emeritus of Harvard Medical School’s M.D./Ph.D. Program since 1996, Chair of Medicine, Emeritus, and Director of the Center for Assessment Technology and Continuous Health (CATCH) at Massachusetts General Hospital, which he co-founded, since 2012, and
8
Physician-in-Chief Emeritus at Massachusetts General Hospital since 2013. From 1996 to April 2013, Dr. Ausiello served as the Chief of Medicine at Massachusetts General Hospital. Dr. Ausiello is a member of the Institute of Medicine of the National Academy of Sciences and a fellow of the American Academy of Arts and Sciences. Dr. Ausiello has served on the boardsboard of directors of Alnylam Pharmaceuticals since April 2012 and Rani Therapeutics Holdings, Inc.as Vice Chairman of the board of directors of Spexis AG, a clinical-stage biopharmaceutical company, since September 2018,December 2021, and previously served on the board of directors of Pfizer Inc. from 2006 to 2019,2020, where he currently serves on the advisory board since 2019. Dr. Ausiello also serves on the boards of directors of numerous privately held companies. Dr. Ausiello received a B.A. in Biochemistry from Harvard College and an M.D. from the University of Pennsylvania. We believe that Dr. Ausiello is qualified to serve on our Board of Directors because of his extensive experience as a physician and as a director of pharmaceutical companies.
STEPHEN A. BERENSON | Age 63 |
Stephen A. Berenson has served as Chairman of our Board of Directors since December 2019 and as a member of our Board of Directors since August 2019. Mr. Berenson has been a Managing Partner at Flagship Pioneering, a life sciences innovation firm which conceives, creates, resources and develops first-in-category bioplatform companies, since June 2017. Prior to Flagship, Mr. Berenson spent 33 years in various roles as an investment banker at J.P. Morgan, most recently serving in the role of Vice Chairman of Investment Banking from 2005 to April 2017, where he focused on providing high-touch strategic advice and complex transaction execution to leading companies across all industries globally. He was co-founder of J.P. Morgan’s Global Strategic Advisory Council and co-founder of the firm’s Board Initiative. Mr. Berenson has served as chairman of the board of directors of Cellarity, a privately held pharmaceutical company, since July 2021, and has served on the board of directors of Moderna, Inc., a pharmaceutical and biotechnology company, since October 2017. Mr. Berenson received an S.B. in Mathematics from the Massachusetts Institute of Technology. We believe that Mr. Berenson is qualified to serve on our Board of Directors because of his extensive experience working with rapidly-growing companies across various industries.
WILLARD H. DERE, M.D. | Age |
Willard H. Dere, M.D., has served as a member our Board of Directors since July 2017. Dr. Dere has been Professor Emeritus, Department of Internal Medicine, B. Lueat the University of Utah School of Medicine since July 2022. Prior to retirement, and Hope S. Bettilyon Presidential Endowed Chairbeginning in Internal Medicine for Diabetes Research, and Co-Director of the Clinical and Translational Science InstituteNovember 2014, Dr. Dere held multiple roles at the University of Utah Health Sciences Center, since November 2014 andincluding Associate Vice President for Research, since September 2019.Co-Director of the Utah Clinical and Translational Science Institute, and Co-Director of the Center for Genomic Medicine. Prior to his professorship, from 2003 until his retirement in October 2014, Dr. Dere held multiple rolesworked at Amgen, Inc., including Headwhere he was Senior Vice President and head of Global Development, and both corporate and international Chief Medical Officer,led development programs in multiple therapeutic areas. From 1989 to 2014, he worked at Eli Lilly and led multiple development of programs, and also worked in various therapeutic areas.clinical pharmacology, regulatory affairs and safety. Dr. Dere serveshas served on the boards of directors of several companies, including BioMarin Pharmaceutical, Inc. since July 2016, Radius Health since November 2014, and Mersana Therapeutics, Inc. since March 2018. He also serves2018, and Metagenomi, Inc. since August 2021, and previously served on the boards of directors of privately held companies. From October 2016 to December 2017, he served on the board of directors of Ocera Therapeutics.Therapeutics and Radius Health. Dr. Dere received his B.A. in History and Zoology and M.D. from the University of California, Davis, completed his internal medicine residency training at the University of Utah, and his postdoctoral training in endocrinology and metabolism at the University of California, San Francisco. We believe Dr. Dere is qualified to serve on our Board of Directors due to his extensive academic experience and his knowledge of the biotechnology industry.
CLAIRE M. FRASER, PH.D. | Age 68 |
Claire M. Fraser, Ph.D., has served as a member of our Board of Directors since January 2023. Since 2007, Dr. Fraser has been the director of the Institute for Genome Sciences and a Professor of Medicine and Microbiology and Immunology at the University of Maryland School of Medicine in Baltimore, Maryland. From 1998 to 2007, she served as president and director of The Institute for Genomic Research, a not-for-profit research organization engaged in human and microbial genomics studies. Dr. Fraser has served on the board of directors of Becton, Dickinson, and Company, a medical technology company, since 2006, and previously served as the Chair of the Board and a director of the American Association for the Advancement of Science. Dr. Fraser received her bachelor’s degree in Biology from Rensselaer Polytechnic Institute and her Ph.D. in Pharmacology from State University of New York-Buffalo. We believe Dr. Fraser is qualified to serve on our Board of Directors due to her extensive academic experience and her knowledge of the microbiome industry.
RICHARD N. KENDER | Age 68 |
9
Richard N. Kender has served as a member of our Board of Directors since October 2014. From October 1978 to September 2013, Mr. Kender held positions in a variety of corporate areas at Merck & Co., Inc., or Merck, a pharmaceutical company, most recently serving as Senior Vice President of Business Development and Corporate Licensing. Mr. Kender has served on the boards of directors of Poxel S.A., a clinical stage biopharmaceutical company, since March 2015 and Bicycle Therapeutics PLC since July 2019. He previously served on the boards of directors of INC Research Holdings, Inc. (now known as Syneos Health) between December 2014 and August 2017, Abide Therapeutics, Inc., a privately held company, between December 2015 and May 2019, and ReViral Ltd., a privately held company, from November 2019 to June 2022. Mr. Kender received a B.S. in Accounting from Villanova University and an M.B.A. from Fairleigh Dickinson University. We believe Mr. Kender is qualified to serve on our Board of Directors because of his finance experience and knowledge of the biotechnology industry.
ERIC D. SHAFF | Age |
Eric D. Shaff has served as our President and Chief Executive Officer and a member of our Board of Directors since January 2019. Previously, he served as our Chief Operating and Financial Officer and Executive Vice President from January 2018 until January 2019 and as our Chief Financial Officer from November 2014 until January 2019. From January 2012 to November 2014, Mr. Shaff was Vice President of Corporate Finance for Momenta Pharmaceuticals, or Momenta, a biotechnology company, where he helped manage Momenta’s accounting, finance, planning, and procurement functions, as well as contributing to Momenta’s investor relations efforts. Prior to Momenta, Mr. Shaff held a number of corporate development and finance positions with Genzyme Corporation, a biotechnology company, most recently as Vice President of Finance/Controller for the Personalized Genetic Health division. Mr. Shaff haspreviously served on the board of directors of Sigilon Therapeutics, Inc. since November 2017.from 2017 to August 2023. Mr. Shaff received his B.A. from the University of Pennsylvania and his M.B.A. from Cornell University. We believe Mr. Shaff is qualified to serve on our Board of Directors because of his extensive business and finance experience and his knowledge of the biotechnology industry.
Board Diversity Matrix (as of February 23, 2024) | |||
Board Size (Total Number of Directors): 8 | |||
| Female | Male | Non-Binary |
Part I: Gender Identity | |||
Directors | 1 | 7 | - |
Part II: Demographic Background | |||
Asian | - | 2 | - |
White | 1 | 6 | - |
Two or More Races or Ethnicities | - | 1 | - |
Stephen A. Berenson has served as Chairman of our Board of Directors since December 2019 and as a member of our Board of Directors since August 2019. Mr. Berenson has been a Managing Partner at Flagship Pioneering, a life sciences innovation firm which conceives, creates, resources and develops first-in-category life sciences companies, since June 2017. Prior to Flagship, Mr. Berenson spent 33 years in various roles as an investment banker at J.P. Morgan, most recently serving in the role of Vice Chairman of Investment Banking from 2005 to April 2017, where he focused on providing high-touch strategic advice and complex transaction execution to leading companies across all industries globally. He was co-founder of J.P. Morgan’s Global Strategic Advisory Council and co-founder of the firm’s Board Initiative. Mr. Berenson has served as chairman of the board of directors of Cellarity since July 2021, and has served on the boards of directors of Moderna, Inc. since October 2017 and Repertoire Immune Medicines, a privately held company, since May 2021. Mr. Berenson received an S.B. in Mathematics from the Massachusetts Institute of Technology. We believe that Mr. Berenson is qualified to serve on our Board of Directors because of his extensive experience working with rapidly-growing companies across various industries.
10
Richard N. Kender has served as a member of our Board of Directors since October 2014. From October 1978 to September 2013, Mr. Kender held positions in a variety of corporate areas at Merck & Co., Inc., or Merck, a pharmaceutical company, most recently serving as Senior Vice President of Business Development and Corporate Licensing. Mr. Kender has served on the boards of directors of Poxel S.A. since March 2015, Bicycle Therapeutics PLC since July 2019, and ReViral Ltd, a privately held company, since November 2019. He previously served on the boards of directors of INC Research Holdings, Inc. between December 2014 and August 2017 and Abide Therapeutics, Inc., a privately held company, between December 2015 and May 2019. Mr. Kender received a B.S. in Accounting from Villanova University and an M.B.A. from Fairleigh Dickinson University. We believe Mr. Kender is qualified to serve on our Board of Directors because of his finance experience and knowledge of the biotechnology industry.
Meryl S. Zausner has served as a member of our Board of Directors since August 2018. Ms. Zausner worked for Novartis Pharmaceuticals, Inc., or Novartis, a pharmaceutical company, from 1988 until her retirement in 2017, most recently serving as Chief Financial and Administrative Officer and a member of the Pharmaceutical Executive Committee and Global Finance Leadership Team of Novartis in the United States. At Novartis, she helped launch the Oncology Business Unit, as well as the company’s shared services organization. Prior to serving as Chief Financial and Administrative Officer, Ms. Zausner was a member of the Novartis Global Oncology leadership team, where she contributed to the development and commercialization of therapies, including Gleevec® (imatinib). Ms. Zausner has served on the board of directors of Goldfinch Bio, Inc., a privately held company, since February 2021, and she previously served on the boards of directors of the Multiple Myeloma Research Foundation from September 2009 to June 2021 and Neon Therapeutics, Inc. from December 2017 to May 2020. Ms. Zausner received a B.S. in Accounting and Economics from the University at Albany, SUNY. We believe Ms. Zausner is qualified to serve on our Board of Directors because of her finance and leadership experience and knowledge of the pharmaceutical industry.
Grégory Behar has served as a member of our Board of Directors since December 2014. Mr. Behar has served as Chief Executive Officer of Nestlé Health Science, a business unit of Société des Produits Nestlé S.A., a health sciences company, since July 2014. From August 2011 to May 2014, Mr. Behar was President and Chief Executive Officer of Boehringer Ingelheim Pharmaceuticals Inc. (USA), a pharmaceutical company. From 2010 to July 2011, Mr. Behar was Corporate Vice President Region NECAR (North European Union, Canada and Australasia) for Boehringer-Ingelheim GmbH, a pharmaceutical company. Mr. Behar has served on the boards of directors of Nestlé Health Science since July 2014 and Sonova AG since April 2021 and previously served on the boards of directors of Axcella Health, Inc. from February 2016 to April 2022 and Aimmune Therapeutics, Inc. from November 2016 until its acquisition in October 2020. Mr. Behar received his B.S. in Mechanical Engineering from the University of California, Los Angeles, an M.S. in Mechanical Engineering and Manufacturing from EPFL in Switzerland and an M.B.A. from INSEAD in France. We believe that Mr. Behar is qualified to serve on our Board of Directors because of his extensive business experience in the health sciences and pharmaceutical industries.
Paul R. Biondi has served as a member of our Board of Directors since March 2020. Mr. Biondi is an Executive Partner and President of Pioneering Medicines at Flagship Pioneering, roles he has held since November 2019. Mr. Biondi joined Flagship Pioneering following a seventeen-year tenure at Bristol-Myers Squibb, or BMS, a pharmaceutical company, where he was most recently the Senior
Vice President of Strategy and Business Development from October 2015 to November 2019. Prior to serving in the role of Senior Vice President of Strategy, from 2002 to 2015, Mr. Biondi held a series of other leadership roles within BMS’ Research and Development organization overseeing strategy, portfolio and project management, as well as clinical and business operations. Mr. Biondi holds a bachelor’s degree from Dartmouth College and an M.B.A. from the J.L. Kellogg School of Management at Northwestern University. We believe that Mr. Biondi is qualified to serve on our Board of Directors because of his extensive experience in biopharmaceutical strategy and corporate development.
Kurt C. Graves has served as a member of our Board of Directors since November 2015. Mr. Graves has served as the Executive Chairman of i20 Therapeutics, Inc., a biotechnology company, since August 2021. Mr. Graves was previously the Chairman, President and Chief Executive Officer of Intarcia Therapeutics, Inc., a biotechnology company, from September 2010 to December 2020 and on its board of directors from August 2010 to December 2020. Previously, he served as Executive Vice President, Chief Commercial Officer and Head of Strategic Development at Vertex Pharmaceuticals Inc., or Vertex, from July 2007 to October 2009. Prior to joining Vertex, Mr. Graves held various leadership positions at Novartis Pharmaceuticals Corporation, or Novartis Corp., from 1999 to June 2007, including the Global General Medicines Business Unit Head and Chief Marketing Officer for the pharmaceuticals division of Novartis Corp. from September 2003 to June 2007. He served on the boards of directors of Radius Health, Inc. from May 2011 to March 2020, and Achillion Pharmaceuticals, Inc. from June 2012 to January 2020. Mr. Graves received a B.S. in Biology from Hillsdale College. We believe Mr. Graves is qualified to serve as a member of our Board of Directors because of his extensive experience in the life sciences industry, membership on various boards of directors and his leadership and management experience.
Board Diversity Matrix (as of April 29, 2022) | ||||||
Board Size: | ||||||
Total Number of Directors | 9 | |||||
| Female | Male | Non-Binary | ||||
Part I: Gender Identity | ||||||
Directors | 1 | 8 | - | |||
Part II: Demographic Background | ||||||
Asian | - | 2 | - | |||
White | 1 | 7 | - | |||
Two or More Races or Ethnicities | - | 1 | - | |||
Ratification of Appointment of Independent Registered Public Accounting Firm
The Audit Committee of the Board of Directors (the “Audit Committee”) has appointed PricewaterhouseCoopers LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2022.2024. Our Board of Directors has directed that this appointment be submitted to our stockholders for ratification. Although ratification of our appointment of PricewaterhouseCoopers LLP is not required, we value the opinions of our stockholders and believe that stockholder ratification of our appointment is a good corporate governance practice.
PricewaterhouseCoopers LLP also served as our independent registered public accounting firm for the fiscal year ended December 31, 2021.2023. Neither the accounting firm nor any of its members has any direct or indirect financial interest in or any connection with us in any capacity other than as our auditors, providing audit and non-audit related services. A representative of PricewaterhouseCoopers LLP is expected to attend the Annual Meeting and to have an opportunity to make a statement and be available to respond to appropriate questions from stockholders.
In the event that the appointment of PricewaterhouseCoopers LLP is not ratified by the stockholders, the Audit Committee will consider this fact when it appoints the independent auditors for the fiscal year ending December 31, 2023.2025. Even if the appointment of PricewaterhouseCoopers LLP is ratified, the Audit Committee retains the discretion to appoint a different independent auditor at any time if it determines that such a change is in the interest of our Company.
VOTE REQUIRED
This proposal requires the affirmative vote of the holders of a majority in voting power of the votes cast affirmatively or negatively. Abstentions are not considered to be votes cast and, accordingly, will have no effect on the outcome of the vote on this proposal. Because brokers have discretionary authority to vote on the ratification of the appointment of PricewaterhouseCoopers LLP, we do not expect any broker non-votes in connection with this proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “FOR” the Ratification of the Appointment of PricewaterhouseCoopers LLP as our Independent Registered Public Accounting Firm.
11 |
Approval, on an Advisory (Non-Binding) Basis, of the Compensation of Our Named Executive Officers
In accordance with the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”) and Rule 14a-21 under the Exchange Act, we request that our stockholders cast a non-binding, advisory vote to approve the compensation of our named executive officers identified in the section titled “Executive and Director Compensation” set forth below in this proxy statement. This proposal, commonly known as a “say-on-pay”“say-on-pay” proposal, gives our stockholders the opportunity to express their views on our named executive officers’ compensation. This vote is not intended to address any specific item of compensation, but rather the overall compensation of our named executive officers and the philosophy, policies and practices described in this proxy statement.
Accordingly, we ask our stockholders to vote “FOR” the following resolution at the Annual Meeting:
“RESOLVED, that the Company’s stockholders approve, by a non-binding advisory vote, the compensation of the named executive officers, as disclosed in the Company’s Proxy Statement for the 20222024 Annual Meeting of Stockholders pursuant to the compensation disclosure rules of the Securities and Exchange Commission, including the compensation tables and narrative discussion.”
We believe that our compensation programs and policies for the year ended December 31, 20212023 were an effective incentive for the achievement of our goals, aligned with stockholders’ interest and worthy of stockholder support. Additional details concerning how we structure our compensation programs to meet the objectives of our compensation program are provided in the section titled “Executive Compensation” set forth below in this proxy statement. In particular, we discuss how we design performance-based compensation programs and set compensation targets and other objectives to maintain a close correlation between Company and individual achievement.
This vote is merely advisory and will not be binding upon us, our Board of Directors or our Compensation and Talent Committee, nor will it create or imply any change in the duties of us, our Board of Directors or our Compensation and Talent Committee. The Compensation and Talent Committee will, however, take into account the outcome of the vote when considering future executive compensation decisions. At our 20212023 Annual Meeting of Stockholders, approximately 99.4% of the votes cast on the “say-on-pay”“say-on-pay” proposal were voted “FOR” the proposal. The Board of Directors values constructive dialogue on executive compensation and other significant governance topics with our stockholders and encourages all stockholders to vote their shares on this important matter.
At our 2021 Annual Meeting of Stockholders held on June 16, 2021, our stockholders recommended, on an advisory basis, that the stockholder vote on the compensation of our named executive officers occur every year. In light of the foregoing recommendation, our board of directors determined to hold a “say-on-pay”“say-on-pay” advisory vote every year. Accordingly, our next advisory say-on-pay vote (following the non-binding advisory vote at this Annual Meeting) is expected to occur at the 20232025 Annual Meeting of Stockholders.
VOTE REQUIRED
The approval, on an advisory (non-binding) basis, of the compensation of our named executive officers will require the affirmative vote of the holders of a majority in voting power of the votes cast affirmatively or negatively. Abstentions and broker non-votes are not considered to be votes cast and, accordingly, will have no effect on the outcome of the vote on this proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “FOR” the approval, on an advisory (non-binding) basis, |
Report of the Auditcompensation of our named executive officers.
12
PROPOSAL 4
Approval of an Amendment to Our Restated Certificate of Incorporation to Increase the Number of Authorized Shares of Common Stock
Our Restated Certificate of Incorporation, as amended (our “Restated Certificate of Incorporation”), currently authorizes the issuance of 240,000,000 shares of Common Stock, par value $0.001 per share. On February 22, 2024, our Board adopted a resolution to amend the Restated Certificate of Incorporation, subject to stockholder approval, by increasing the number of authorized shares of our Common Stock to 360,000,000 shares (the “Share Increase Amendment”). The additional 120,000,000 shares of Common Stock authorized for issuance pursuant to the proposed Share Increase Amendment would be part of the existing class of Common Stock and, if and when issued, would have the same rights and privileges as the shares of Common Stock presently issued and outstanding. The holders of Common Stock are not entitled to preemptive rights or cumulative voting.
The Share Increase Amendment will not affect the number of authorized shares of preferred stock of the Company, par value $0.001 per share, which is 10,000,000 shares. Currently, there are no shares of preferred stock issued and outstanding.
If our stockholders approve this proposal, then the first sentence of Article FOURTH of our Restated Certificate of Incorporation will be deleted and replaced in its entirety to read as follows:
“The total number of shares of all classes of stock that the Corporation shall have authority to issue is 370,000,000 shares, consisting of (a) 360,000,000 shares of Common Stock, $0.001 par value per share (“Common Stock”), and (b) 10,000,000 shares of Preferred Stock, $0.001 par value per share (“Preferred Stock”).”
Purpose of Share Increase Amendment
Our Board believes it is in the best interests of the Company and our stockholders to increase our authorized shares of Common Stock in order to have additional shares available for use as our Board deems appropriate or necessary. As such, the primary purpose of the Share Increase Amendment is to provide the Company with greater flexibility with respect to managing its Common Stock in connection with such corporate purposes as may, from time to time, be considered advisable by our Board. These corporate purposes could include, without limitation, financing activities, public or private offerings of Common Stock, stock dividends or splits, conversions of convertible securities, issuance of options and other equity awards pursuant to our incentive plans, establishing a strategic relationship with a corporate collaborator and acquisition transactions. Having an increased number of authorized but unissued shares of Common Stock would allow us to take prompt action with respect to corporate opportunities that develop, without the delay and expense of convening a special meeting of stockholders for the purpose of approving an increase in our capitalization. Our Board will determine whether, when and on what terms the issuance of shares of Common Stock may be warranted in connection with any of the foregoing purposes.
Effect of Approval of Proposed Amendment
The following table illustrates the effect the proposed Share Increase Amendment would have on the number of shares of Common stock available for issuance, if approved by our stockholders:
| As of February 21, 2024 |
|
| Upon Effectiveness of Amendment |
| ||
TOTAL AUTHORIZED SHARES OF COMMON STOCK |
| 240,000,000 |
|
|
| 360,000,000 |
|
Outstanding shares of Common Stock |
| 147,464,462 |
|
|
| 147,464,462 |
|
Shares of Common Stock authorized for future issuance under the Company’s incentive plans |
| 3,338,234 |
|
|
| 3,338,234 |
|
Shares of Common Stock authorized for future issuance under the Company’s employee stock purchase plan |
| 2,666,512 |
|
|
| 2,666,512 |
|
Shares of Common Stock subject to outstanding equity awards under the Company’s incentive plans |
| 17,723,433 |
|
|
| 17,723,433 |
|
Shares of Common Stock issuable upon exercise of outstanding warrants |
| 1,177,433 |
|
|
| 1,177,433 |
|
Total outstanding shares of Common Stock and shares of Common Stock Reserved |
| 172,370,074 |
|
|
| 172,370,074 |
|
Unreserved shares of Common Stock available for issuance(1) |
| 67,629,927 |
|
|
| 187,629,927 |
|
(1) Includes shares of Common Stock, if any, that may be issued under the “at the market" equity offering program.
Other than shares that will be reserved for issuance under our existing incentive plans, employee stock purchase plan, and "at the market" equity offering program, we do not currently have any arrangements, agreements or understandings that would require the issuance of additional shares of Common Stock. Because our directors and executive officers have outstanding equity awards under our incentive plans, and may be granted additional equity awards under these plans, they may be deemed to have an indirect interest in the Share Increase Amendment because, absent the amendment, the Company may not have sufficient authorized shares to make future awards.
13
The Share Increase Amendment will not have any immediate effect on the rights of existing stockholders. However, our Board will have the authority to issue authorized Common Stock without requiring future stockholder approval of such issuances, except as may be required by applicable law or rules of the Nasdaq Stock Market. Future issuances of Common Stock or securities convertible into or exchangeable for Common Stock could have a dilutive effect on our earnings per share, book value per share and the voting power and interest of current stockholders.
If the Share Increase Amendment is approved by stockholders, all other sections of the Restated Certificate of Incorporation would be maintained in their current form. The Share Increase Amendment would become effective upon the filing of a Certificate of Amendment to our Restated Certificate of Incorporation with the Secretary of State of the State of Delaware, which the Company intends to do promptly after the Annual Meeting if this Proposal is approved by stockholders. In the event that the Share Increase Amendment is not approved by our stockholders at the Annual Meeting, the current Restated Certificate of Incorporation would remain in effect in its entirety. Our Board reserves the right, notwithstanding stockholder approval of the Share Increase Amendment and without further action by our stockholders, not to proceed with the Share Increase Amendment at any time before it becomes effective.
Potential Anti-Takeover Effect
Our Board has not proposed the Share Increase Amendment with the intention of discouraging tender offers or takeover attempts of the Company. However, the availability of additional authorized shares for issuance could, under certain circumstances, discourage or make more difficult efforts to obtain control of our company. This proposal is not being presented with the intent that it be used to prevent or discourage any acquisition attempt, but nothing would prevent our Board from taking any appropriate actions not inconsistent with its fiduciary duties.
Dissenters’ Rights of Appraisal
Under Delaware law, stockholders are not entitled to appraisal rights with respect to the Share Increase Amendment, and we will not independently provide our stockholders with any such right.
VOTE REQUIRED
The approval of the Share Increase Amendment will require the affirmative vote of the holders of a majority of the stock of the Company entitled to vote at the Annual Meeting. Abstentions will have the same effect as votes against this Proposal. Because brokers have discretionary authority to vote on this Proposal, we do not expect any broker non-votes in connection with this Proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “FOR” the approval of an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of common stock.
14
PROPOSAL 5
Approval of an Adjournment of the Annual Meeting
Our stockholders are being asked to approve an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4.
VOTE REQUIRED
The approval of an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4 will require the affirmative vote of the holders of a majority of voting power of the votes cast affirmatively or negatively. Abstentions will have no effect on this Proposal. Because brokers have discretionary authority to vote on this Proposal, we do not expect any broker non-votes in connection with this Proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “FOR” the approval of an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4.
15
PROPOSAL 6
Stockholder Proposal on Simple Majority Vote
Kenneth Steiner, whose listed address is 14 Stoner Avenue, 2M, Great Neck, New York 11021, has notified us of his intent to present the following proposal at the Annual Meeting. The stockholder proposal is required to be voted upon at our Annual Meeting only if properly presented by or on behalf of Mr. Steiner.Mr. Steiner has provided his proxy for John Chevedden to act on Mr. Steiner’s behalf with respect to the proposal.We are not responsible for the accuracy or content of the proposal or supporting statement, which are presented below as received from the stockholder proponent in accordance with SEC rules.We will promptly provide you with the number of voting securities held by Mr. Steiner, to our knowledge, upon receiving a written or oral request at c/o Secretary, Seres Therapeutics, Inc., 101 Cambridgepark Drive, Cambridge, MA 02140, or by phone at: (617) 945-9626.
If properly presented at the Annual Meeting by or on behalf of the stockholder proponent, our Board of Directors opposes and unanimously recommends that you vote “AGAINST” the Stockholder Proposal for the reasons stated under “Statement in Opposition to the Stockholder Proposal.”
STOCKHOLDER PROPOSAL
Proposal 6 – Simple Majority Vote

Shareholders request that our board take each step necessary so that each voting requirement in our charter and bylaws (that is explicit or implicit due to default to state law) that calls for a greater than simple majority vote be replaced by a requirement for a majority of the votes cast for and against applicable proposals, or a simple majority in compliance with applicable laws. If necessary this means the closest standard to a majority of the votes cast for and against such proposals consistent with applicable laws. This includes making the necessary changes in plain English.
Shareholders are willing to pay a premium for shares of companies that have excellent corporate governance. Supermajority voting requirements have been found to be one of 6 entrenching mechanisms that are negatively related to company performance according to "What Matters in Corporate Governance" by Lucien Bebchuk, Alma Cohen and Allen Ferrell of the Harvard Law School. Supermajority requirements are used to block initiatives supported by most shareowners but opposed by a status quo management.
This proposal topic won from 74% to 88% support at Weyerhaeuser, Alcoa, Waste Management, Goldman Sachs, FirstEnergy, McGraw-Hill and Macy’s. These votes would have been higher than 74% to 88% if more shareholders had access to independent proxy voting advice. This proposal topic also received overwhelming 98%-support each at the 2023 annual meetings of American Airlines (AAL) and The Carlyle Group (CG).
Please vote yes:
Simple Majority Vote – Proposal 6.
STATEMENT IN OPPOSITION TO THE STOCKHOLDER PROPOSAL
This stockholder proposal broadly requests that every voting requirement in our Restated Certificate of Incorporation or Amended and Restated Bylaws that calls for a greater than simple majority vote, regardless of what the voting requirement is or what it addresses, be replaced by a blanket “majority of vote cast” standard or “a simple majority in compliance with applicable laws”. The Board has given careful consideration to the stockholder proposal and, for the following reasons, does not believe that this stockholder proposal would enhance the Company’s corporate governance or be in the best interests of the Company or its stockholders.
Existing Supermajority Voting Thresholds Apply in Limited Circumstances
Apart from the election of directors, the Company’s current governance documents require the vote of a majority of the shares present and entitled to vote for all matters submitted for stockholder approval, except in certain limited circumstances, including approving the removal of directors or modifying the Amended and Restated Bylaws or certain sections of the Company’s Restated Certificate of Incorporation. Under the proposed standard, however, only a “majority
16
of the votes cast” or “a simple majority in compliance with applicable laws” would be required to approve such fundamental corporate actions.
The Board believes that in certain limited circumstances, the higher voting requirements are appropriate and desirable, because certain fundamental matters should require the support of a broad consensus of the Company’s stockholders, rather than a simple majority of the votes present at a meeting. The Board believes that the limited supermajority requirements the Company has in place are appropriate to maintain the stability of our operations, while striking an appropriate balance that allows for fundamental changes where there is strong stockholder consensus. In this manner, supermajority thresholds also assist in maximizing long-term value to all stockholders and have the effect of deterring hostile takeovers of our Company that may not be in the best interests of the Company and our stockholders.
Existing Supermajority Voting Thresholds Benefit a Wide Base of Stockholders
The stockholder proposal’s request to eliminate all supermajority provisions would leave our stockholders vulnerable to self-interested and potentially abusive actions proposed by small groups of stockholders who hold a large number of shares and who may seek to, and with a simple majority standard be more easily able to, advance their own interests over the interests of all of the Company’s other stockholders.
For example, if the stockholder proposal were implemented and a quorum of 50.1% of the Company’s outstanding shares was present at a stockholders meeting, certain fundamental matters, such as amending the Company’s Amended and Restated Bylaws, could be adopted by stockholders who hold 25.1% of the Company’s outstanding shares. As of February 1, 2024, based on publicly available information filed pursuant to Section 13 of the Exchange Act, four of the Company’s largest stockholders held an aggregate of approximately 40% of the Company’s outstanding shares. If this stockholder proposal were implemented, it would be possible for just two of our largest stockholders to, without any other stockholder support, approve a proposal amending our Amended and Restated Bylaws. As such, if this stockholder proposal is approved, a very small group of stockholders, who are not bound by a fiduciary duty to act in the best interests of the Company and its stockholders, would have outsized power to act in their own respective self-interests, potentially to the detriment of the Company and the Company’s other stockholders.
Our Current Governance Structure Promotes Effective Board Oversight
The stockholder proposal is both vague and overbroad in that it does not specify which provisions that the stockholder proponent is seeking to change. The Board and the Nominating and Corporate Governance Committee of the Board of Directors (the “Nominating and Corporate Governance Committee”) do not believe that indiscriminate elimination of all supermajority voting requirements and the imposition of a blanket “majority of votes cast” or “simple majority in compliance with applicable laws” standard, without regard for how such a voting requirement would apply to each provision it impacts, or how that, in turn, would impact the Company’s stockholders, would be in the best interests of our stockholders. Our Board is committed to effective corporate governance and has adopted a wide range of practices and procedures that promote effective Board oversight. For example:
17
Consistent with its current practice, the Board will continue to evaluate the future implementation of appropriate corporate governance changes.
However, the Board strongly urges an AGAINST vote on this stockholder proposal that risks:
For these reasons, the Board does not believe it is in the best interests of stockholders to implement the stockholder proposal’s request.
VOTE REQUIRED
The approval of the stockholder proposal on simple majority vote will require the affirmative vote of the holders of a majority of voting power of the votes cast affirmatively or negatively. Abstentions and broker non-votes are not considered to be votes cast and, accordingly, will have no effect on the outcome of the vote on this proposal.
RECOMMENDATION OF THE BOARD OF DIRECTORS
The Board of Directors unanimously recommends a vote “AGAINST” the stockholder proposal on simple majority vote.
18
Report of the Audit Committee of the Board of Directors
The Audit Committee has reviewed the audited consolidated financial statements of the Company for the fiscal year ended December 31, 20212023 and has discussed these financial statements with management and the Company’s independent registered public accounting firm. The Audit Committee has also received from, and discussed with, the Company’s independent registered public accounting firm the matters that they are required to provide to the Audit Committee, including the matters required to be discussed by the Public Company Accounting Oversight Board (“PCAOB”) and the SEC.
The Company’s independent registered public accounting firm also provided the Audit Committee with a formal written statement required by PCAOB Rule 3526 (Communications with Audit Committees Concerning Independence) describing all relationships between the independent registered public accounting firm and the Company, including the disclosures required by the applicable requirements of the PCAOB regarding the independent registered public accounting firm’s communications with the Audit Committee concerning independence. In addition, the Audit Committee discussed with the independent registered public accounting firm its independence from the Company.
Based on its discussions with management and the independent registered public accounting firm, and its review of the representations and information provided by management and the independent registered public accounting firm, the Audit Committee recommended to the Board of Directors that the audited consolidated financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021.2023.
Richard N. Kender (Chair)
Claire M. Fraser, Ph.D.
Willard H. Dere, M.D.
19
Meryl S. Zausner
Independent RegisteredRegistered Public Accounting Firm Fees and Other Matters
The following table summarizes the fees of PricewaterhouseCoopers LLP, our independent registered public accounting firm, billed to us for each of the last two fiscal years for audit services and billed to us in each of the last two fiscal years for other services:
Fee Category | 2021 | 2020 |
| 2023 |
|
| 2022 |
| ||||||||
Audit Fees | $ | 1,640,000 | $ | 896,000 |
| $ | 1,695,000 |
|
| $ | 1,690,000 |
| ||||
Audit-Related Fees | $ | 50,000 | $ | 50,000 |
| $ | — |
|
| $ | 50,000 |
| ||||
Tax Fees | $ | — | $ | — |
| $ | — |
|
| $ | — |
| ||||
All Other Fees | $ | 2,756 | $ | 2,756 |
| $ | 568,980 |
|
| $ | 4,409 |
| ||||
Total Fees | $ | 1,692,756 | $ | 948,756 |
| $ | 2,263,980 |
|
| $ | 1,744,409 |
| ||||
AUDIT FEES
Audit Fees
Audit fees in 20212023 and 20202022 consist of fees billed for the audit of our annual consolidated financial statements, the review of the interim consolidated financial statements, and related services that are normally provided in connection with registration statements, and services performed in connection with our at the market common stock offering and registered direct offering.
AUDIT-RELATED FEES
Audit-Related Fees
Audit-related fees in 2021 and 20202022 consist of fees in connection with an audit of our grant from CARB-X.
TAX FEES
There were no such fees incurred in 20212023.
Tax Fees
There were no such fees incurred in 2023 or 2020.2022.
ALL OTHER FEES
All Other Fees
All other fees in 20212023 and 20202022 represent non-audit fees in connection with access to the PricewaterhouseCoopers LLP on-line accounting research and disclosures database.database and other advisory and consulting services.
AUDIT COMMITTEE PRE-APPROVAL POLICY AND PROCEDURES
Audit Committee Pre-Approval Policy and Procedures
The Audit Committee has adopted a policy (the “Pre-Approval“Pre-Approval Policy”) which sets forth the procedures and conditions pursuant to which audit and non-audit services proposed to be performed by the independent auditor may be pre-approved. The Pre-Approval Policy generally provides that we will not engage PricewaterhouseCoopers LLP to render any audit, audit-related, tax or permissible non-audit service unless the service is either (i) explicitly approved by the Audit Committee (“specific pre-approval”) or (ii) entered into pursuant to the pre-approval policies and procedures described in the Pre-Approval Policy (“general pre-approval”). Unless a type of service to be provided by PricewaterhouseCoopers LLP has received general pre-approval under the Pre-Approval Policy, it requires specific pre-approval by the Audit Committee or by a designated member of the Audit Committee to whom the committee has delegated the authority to grant pre-approvals. Any proposed services exceeding pre-approved cost levels or budgeted amounts will also require specific pre-approval. For both types of pre-approval, the Audit Committee will consider whether such services are consistent with the SEC’s rules on auditor independence. The Audit Committee will also consider whether the independent auditor is best positioned to provide the most effective and efficient service, for reasons such as its familiarity with our business, people, culture, accounting systems, risk profile and other factors, and whether the service might enhance our ability to manage or control risk or improve audit quality. All such factors will be considered as a whole, and no one factor should necessarily be determinative. On an annual basis, the Audit Committee reviews and generally
pre-approves the services (and related fee levels or budgeted amounts) that may be provided by PricewaterhouseCoopers LLP without first obtaining specific pre-approval from the Audit Committee. The Audit Committee may revise the list of general pre-approved services from time to time, based on subsequent determinations.
20
The following table identifies our current executive officers:
Name | Age | Position | ||
| ||||
Eric D. Shaff(1) | 48 | President and Chief Executive Officer | ||
David Arkowitz(2) | 62 | Executive Vice President, Chief Financial Officer and Head of Business Development | ||
| ||||
Thomas J. DesRosier | 69 | Executive Vice President and Chief Legal Officer | ||
David S. Ege, Ph.D. | 49 | Executive Vice President and Chief Technology Officer | ||
Matthew Henn, Ph.D. | 49 | Executive Vice President and Chief Scientific Officer | ||
Lisa von Moltke, M.D. | 65 | Executive Vice President and Chief Medical Officer | ||
Teresa L. Young, Ph.D. | 57 | Executive Vice President, Chief Commercial and Strategy Officer |
|
21
|
|
|
|
|
|
|
GENERAL
Our Board of Directors has adopted Corporate Governance Guidelines, an Insider Trading Compliance Policy, a Code of Business Conduct and Ethics, a Clawback Policy (as defined below) and charters for the Nominating and Corporate Governance Committee, of the Board of Directors (the “Nominating and Corporate Governance Committee”), the Audit Committee, and the Compensation and Talent Committee of the Board of Directors (the “Compensation and Talent Committee”), and the Science and Clinical Development Committee of the Board of Directors (the "Science and Clinical Development Committee") to assist the Board in the exercise of its responsibilities and to serve as a framework for the effective governance of our Company. You can access our current committee charters, our Corporate Governance Guidelines, Insider Trading Compliance Policy, and our Code of Business Conduct and Ethics in the “Corporate Governance” section of the “Investors and News” page of our website located at www.serestherapeutics.com, or by writing to our Secretary at our offices at 200 Sidney Street,101 Cambridgepark Drive, Cambridge, MA 02139.
BOARD COMPOSITION
Our Board of Directors currently consists of nineeight members: Eric D. Shaff, Dennis A. Ausiello, M.D., Grégory Behar, Stephen A. Berenson, Paul R. Biondi, Willard H. Dere, M.D., Claire M. Fraser, Ph.D., Richard N. Kender, and Kurt C. Graves and Meryl S. Zausner.Graves. As set forth in our Restated Certificate of Incorporation, the Board of Directors is currently divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our Restated Certificate of Incorporation and Amended and Restated Bylaws provide that the authorized number of directors may be changed only by resolution of the Board of Directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our Board of Directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of our Company. Our directors may be removed only for cause by the affirmative vote of the holders of at least two-thirds in voting power of the outstanding shares of our capital stock entitled to vote in the election of directors.
DIRECTOR INDEPENDENCE
All of our current directors, other than Eric D. Shaff, qualify as “independent” in accordance with the listing requirements of The Nasdaq Global Select Market (“Nasdaq”). Grégory Behar and Meryl S. Zausner qualified as independent during the period they served on the Board of Directors until their departures on October 2, 2023 and June 22, 2023, respectively. The Nasdaq independence definition includes a series of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by Nasdaq rules, our Board of Directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our Board of Directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management, including, as to each of Mr. Berenson and Mr. Biondi, his relationship to, and our transactions with, Flagship Pioneering and its affiliates, and as to Mr. Behar, during the period that he served, his relationship to, and our transactions with, Nestlé Health Science US Holdings, Inc.S.A. and Société des Produits Nestlé S.A.its affiliates. Mr. Shaff is not independent because he is the President and Chief Executive Officer of our Company. There are no family relationships among any of our directors or executive officers.
DIRECTOR CANDIDATES
The Nominating and Corporate Governance Committee is primarily responsible for searching for qualified director candidates for election to the Board and filling vacancies on the Board. To facilitate the search process, the Nominating and Corporate Governance Committee may solicit current directors and executives of the Company for the names of potentially qualified candidates or ask directors and executives to pursue their own business contacts for the names of potentially qualified candidates. The Nominating and Corporate Governance Committee may also consult with outside advisors or retain search firms to assist in the search for qualified candidates, or consider director candidates recommended by our stockholders. Once potential candidates are identified, the Nominating and Corporate Governance Committee reviews the backgrounds of those candidates, evaluates candidates’ independence from the Company and potential conflicts of interest and determines if candidates meet the qualifications desired by the Nominating and Corporate Governance Committee for candidates for election as a director.
22
In evaluating the suitability of individual candidates (both new candidates and current Board members), the Nominating and Corporate Governance Committee, in recommending candidates for election, and the Board, in approving (and, in the case of vacancies, appointing) such candidates, may take into account many factors, including: personal and professional integrity, ethics and values; experience in corporate management, such as serving as an officer or former officer of a publicly held company; strong finance experience; experience relevant to the Company’s industry; experience as a board member or executive officer of another publicly held company; relevant academic expertise or other proficiency in an area of the Company’s operations; diversity of expertise and experience in substantive matters pertaining to the Company’s business relative to other board members; diversity of background and perspective, including, but not limited to, with respect to age, gender, race, place of residence and specialized experience; practical and mature business judgment, including, but not limited to, the ability to make independent analytical inquiries; and any other relevant qualifications, attributes or skills. The Board evaluates each individual in the context of the Board as a whole, with the objective of assembling a group that can best perpetuate the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas. In determining whether to recommend a director for re-election, the Nominating and Corporate Governance Committee may also consider the director’s past attendance at meetings and participation in and contributions to the activities of the Board.
Stockholders may recommend individuals to the Nominating and Corporate Governance Committee for consideration as potential director candidates by submitting the names of the recommended individuals, together with appropriate biographical information and background materials, to the Nominating and Corporate Governance Committee, c/o Secretary, Seres Therapeutics, Inc., 200 Sidney Street,101 Cambridgepark Drive, Cambridge, MA 02139.02140. In the event there is a vacancy, and assuming that appropriate biographical and background material has been provided on a timely basis, the Nominating and Corporate Governance Committee will evaluate stockholder-recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others.
COMMUNICATIONS FROM STOCKHOLDERS
The Board will give appropriate attention to written communications that are submitted by stockholders, and will respond if and as appropriate. Our Secretary is primarily responsible for monitoring communications from stockholders and for providing copies or summaries to the directors as he considers appropriate.
Communications are forwarded to all directors if they relate to important substantive matters and include suggestions or comments that our Secretary and Chairman of the Board consider to be important for the directors to know. In general, communications relating to corporate governance and long-term corporate strategy are more likely to be forwarded than communications relating to ordinary business affairs, personal grievances and matters as to which we tend to receive repetitive or duplicative communications.
Stockholders who wish to send communications on any topic to an individual director or to the Board should address such communications to such director or to the Board of Directors in writing: c/o Secretary, Seres Therapeutics, Inc., 200 Sidney Street,101 Cambridgepark Drive, Cambridge, MA 02139.02140.
BOARD LEADERSHIP STRUCTURE AND ROLE IN RISK OVERSIGHT
Our Amended and Restated Bylaws and Corporate Governance Guidelines provide our Board of Directors with flexibility to combine or separate the positions of Chairman of the Board and Chief Executive Officer in accordance with its determination that utilizing one or the other structure would be in the best interests of our Company based on the circumstances at that time. We recognize that different board leadership structures may be appropriate for companies in different situations.
Based on the Company’s present circumstances, the Board believes that the Company and its stockholders are best served by having Mr. Berenson serve as its Chairman of the Board and Mr. Shaff serve as its Chief Executive Officer. Our current leadership structure permits Mr. Shaff to focus his attention on managing our Company and permits Mr. Berenson to manage the Board. We believe that this governance structure best reinforces the independence of the Board from management. In addition, we believe the Chairman is well-positioned to act as a bridge between management and the Board, facilitating the regular flow of information. Among other duties, the Chairman of the Board may represent the Board in communications with stockholders and other stakeholders and provide input on the structure and composition of the Board. Accordingly, we believe our current leadership structure is the optimal structure for us at this time.
However, our Board of Directors will continue to periodically review our leadership structure and may make such changes in the future as it deems appropriate. During its routine review of the Board’s leadership structure, the Board and the Company regularly consider the circumstances under which the roles of Chairman and Chief Executive Officer could most effectively serve the Company’s and its stockholders’ interests if combined. From time to time, the Company proactively engages with stockholders throughout the year to learn their perspectives on significant issues, and intends to continue to
23
do so, including with respect to gathering stockholder perspectives on Board leadership structure. If, in the future, the Chairman of the Board is a member of management or does not otherwise qualify as independent, our Corporate Governance Guidelines provide for the appointment by the independent directors of a Lead Director. TheIf appointed, the Lead Director’s responsibilities would include, but would not be limited to, presiding over all meetings of the Board of Directors at which the Chairman of the Board is not present, including any executive sessions of the independent directors, approving the Board’s meeting schedules and agendas, and acting as liaison between the independent directors of the Board and the Chief Executive Officer and the Chairman of the Board.
Risk assessment and oversight are an integral part of our governance and management processes. Our Board of Directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. ManagementManagement’s involvement in day-to-day risk management enables the Company’s disclosure committee, which consists of members of management, to oversee our Chief Executive Officer and Chief Financial Officer in the effective design, establishment, maintenance, review, and evaluation of the Company’s disclosure controls and procedures. The Company’s management, led by our Chief Executive Officer and executive team, implements and supervises day‑to‑day risk management processes. Additionally, management discusses strategic and operational risks at regular management meetings and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the Board of Directors at regular Board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our Board of Directors does not have a standing risk management committee, but rather administers this oversight function directly through the Board of Directors as a whole, as well as through various standing committees of the Board of Directors that address risks inherent in their respective areas of oversight. In particular, our Board of Directors is responsible for monitoring and assessing strategic risk exposure, including business continuity risks, such as risks relating to the COVID-19 pandemic, and our Audit Committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. In addition, our Audit Committee has the responsibility to consider the adequacy and effectiveness of our information security policies and practices, including those concerning data privacy and cybersecurity. The Audit Committee receives quarterly reports from our Chief Information Officer, or CIO, on our cybersecurity risks, and our CIO updates the Audit Committee, as necessary, regarding any material cybersecurity incidents, as well as any incidents with lesser impact potential. The Audit Committee also monitors compliance with legal and regulatory requirements and considers and approves or disapproves any related person transactions. Our Nominating and Corporate Governance Committee oversees risks associated with our corporate governance framework and monitors the effectiveness of our environmental, social and corporate governance (“ESG”) strategy and the Corporate Governance Guidelines. Our Compensation and Talent Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. The allocation of risk oversight among the Board and its committees is consistent with the Board’s leadership structure.
ENVIRONMENTAL, SOCIAL AND CORPORATE GOVERNANCE ACTIVITIES
environmental, social and corporate governance ACTIVITIES
We are committed to policies and practices focused on ESG matters, which commitment isare shaped by our core values and aim to make a positive impact in our communities.the communities where we work and live. We have a history and culture of community service and continue to be involved in, and supportive of, Life Science Cares and Special Olympics Massachusetts. One of the 2021 initiatives that weWe are mostalso proud of isto continue a student education outreach program, Seres Scholars, which is a partnership with the Cambridge Rindge & Latin School to promote equity in education by providing career mentorship, guidance, and opportunities for underserved students from our community. The program values and celebrates diversity, equity, inclusion, and inclusion,belonging ("DEIB") by seeking to include students from all backgrounds, and awards high school seniors with a Seres scholarship after a year and a half of participation. Additionally, we encourage employee volunteerism in all Seres locations by planning activities through our Seres Community Ambassadors program and offering eight hours of paid volunteer time annually for employees to support charitable organizations.
We also believe that our long-term success and ability to deliver innovative, safe, and effective medicines to patients requires a diverse andan inclusive workforce. We value diversity at all levels of the organization and continue to focus on extending our diversity, equity and inclusionDEIB initiatives across our entire workforce, including by working with managersworkforce. We have a dedicated DEIB workstream to develop strategies for building diverse, high performing teams; workingidentify ways to attract, develop, and retain diverse talent from all backgrounds; increasingbackgrounds, increase awareness within our company of unconscious biases;biases, and supporting employee affinity groups comprisedhelp foster a stronger sense of individuals who are underrepresented in our company, industry or society, such as women, members of the LGBTQ+ community and people of color.belonging for all employees. In addition, we strive to engender an open culture of mutual respect, among co-workers,and one that values employees’ health and well-being and fosters professional development.well-being. We support employee growth and development in a variety of ways including with groupleadership training individual mentoringto build people manager capabilities, ongoing performance and coaching, conference attendancedevelopment conversations, and tuition reimbursement. Our management conducts annual employee engagement surveys and reports to our board of directors
24
on human capital management topics, including as relevantrelevant: corporate culture, diversity, equity and inclusion,DEIB, employee development and retention, and compensation and benefits.
ANNUAL BOARD EVALUATION
Our Corporate Governance Guidelines require the Nominating and Corporate Governance Committee to oversee an annual assessment of the Board and its committees. Our outside counsel, Latham & Watkins, LLP (“Latham”), distributed board evaluations to our Board and each Board member returned an evaluation anonymously. Latham tabulated the results and presented them to the Nominating and Corporate Governance Committee for review and discussion.
CODE OF ETHICS
We have a written Code of Business Conduct and Ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer, and controller, or persons performing similar functions. We have posted a current copy of the code on our website, www.serestherapeutics.com. In addition, we intend to post on our website all disclosures that are required by law or the rules of Nasdaq concerning any amendments to, or waivers from, any provision of the code.
ANTI-HEDGING AND ANTI-PLEDGING POLICY
Our Board of Directors has adopted an Insider Trading Compliance Policy, which applies to all of our directors, officers and employees. The policy prohibits our directors, officers and employees and any entities they control from purchasing financial instruments such as zero-costprepaid variable forward contracts, equity swaps, collars, and forward sale contracts,exchange funds, or otherwise engaging in transactions that hedge or offset, or are designed to hedge or offset, any decrease in the market value of the Company’s equity securities, or that may cause an officer, director, or employee to no longer have the same objectives as the Company’s other stockholders. In addition, individuals subject to this policy are prohibited from pledging the Company’s securities as collateral to secure loans.
Our Board of Directors has adopted a Policy for Recovery of Erroneously Awarded Compensation (the "Clawback Policy"), in accordance with the Nasdaq listing standards and Rule 10D-1 under the Exchange Act, which applies to our current and former executive officers. Under the Clawback Policy, we are required to recoup the amount of any erroneously awarded compensation (as defined in the Clawback Policy) on a pre-tax basis within a specified lookback period in the event of any accounting Restatement (as defined in the Clawback Policy), subject to limited impracticability exceptions. Covered restatements include both a restatement to correct an error that is material to previously issued financial statements or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period. The amount required to be recovered is the excess of the amount of incentive-based compensation received over the amount that otherwise would have been received had it been determined based on the restated financial measure. The Clawback Policy is overseen and administered by the Compensation and Talent Committee.
ATTENDANCE BY MEMBERS OF THE BOARD OF DIRECTORS AT MEETINGS
There were eightfour meetings of the Board of Directors during the fiscal year ended December 31, 2021.2023. During the fiscal year ended December 31, 2021,2023, each director attended at least 80%75% of the aggregate of all meetings of the Board of Directors and meetings of the committees on which the Director served during the period in which he or she served as a director.
Currently, we do not maintain a formal policy regarding director attendance at the Annual Meeting; however, it is expected that directors will attend absent compelling circumstances. All of our then-incumbent directors attended our annual meeting of stockholders held in 2021.2023.
25
Our Board has established threefour standing committees—Audit, Compensation and Talent, and Nominating and Corporate Governance—Governance, and Science and Clinical Development—each of which operates under a written charter that has been approved by our Board.Board and is available on our website at www.serestherapeutics.com. All of the members of each of the Board’s threefour standing committees are independent as defined under the Nasdaq rules. Our Board of Directors has determined that Richard N. Kender, Willard H. Dere, M.D., and Meryl S. ZausnerClaire M. Fraser, Ph.D., meet the independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All members of the Compensation and Talent Committee meet the heightened standard for independence specific to members of a compensation committee under the Nasdaq rules and each qualifies as a “non-employee“non-employee director” as defined in Rule 16b-3 of the Exchange Act. All members of the Nominating and Corporate Governance Committee are independent under the Nasdaq rules.
The members of each of the Board committees and committee Chairs are set forth in the following chart.
Name | Audit | Compensation and Talent | Nominating and Corporate Governance |
| ||
Dennis A. Ausiello, M.D. | X | Chair | ||||
Stephen A. Berenson | Chair | |||||
Paul R. Biondi | X | |||||
| ||||||
Willard H. Dere, M.D. | X | X | ||||
Claire M. Fraser, Ph.D. | X | X | ||||
Kurt C. Graves | Chair | |||||
Richard N. Kender | Chair | |||||
| X |
AUDIT COMMITTEE
Our Audit Committee’s responsibilities include:
•
•
•
•
•
•
•
•
•
The members of the Audit Committee are Dr. Dere, Dr. Fraser, and Mr. Kender and Ms. Zausner.Kender. Mr. Kender serves as the Chairperson of the committee. Ms. Zausner served as a member of the Audit Committee until June 2023. The members of our Audit Committee meet the requirements for financial literacy under the applicable rules of Nasdaq. Our Board of Directors has determined that each of Dr. Dere, Dr. Fraser, and Mr. Kender and Ms. Zausner is an “audit committee financial expert” as defined by Item 407(d)(5)(ii) of Regulation S-K.
The Audit Committee met sixfive times in 2021.2023.
26
COMPENSATION AND TALENT COMMITTEE
Our Compensation and Talent Committee is responsible for assisting the Board in the discharge of its responsibilities relating to the compensation of our executive officers, as well as the evaluation, talent development and succession planning for our executive officers and other senior executives. In fulfilling its purpose, our Compensation and Talent Committee has the following principal duties:
•
•
•
•
•
•
•
•
•
•
•
The Compensation and Talent Committee has the authority to retain or obtain the advice of compensation consultants, legal counsel and other advisors to assist in carrying out its responsibilities. For information regarding the role of our compensation consultants in determining our executive compensation, please refer to the section entitled “Executive and Director Compensation— Compensation Setting Process.”
The Compensation and Talent Committee may delegate its authority under its charter to one or more subcommittees as it deems appropriate from time to time as further described in its charter, which is available on our website at www.serestherapeutics.com.charter. The Compensation and Talent Committee may also delegate to an officer the authority to grant equity awards to certain employees, as further described in its charter and subject to the terms of our equity plans.
The members of our Compensation and Talent Committee are Messrs. Biondi, Graves and KenderKender. Ms. Zausner served as a member of the Compensation and Ms. Zausner.Talent Committee until June 2023. Mr. Graves serves as the Chairperson of the Compensation and Talent Committee.
The Compensation and Talent Committee met sixfive times in 2021.
NOMINATING AND CORPORATE GOVERNANCE COMMITTEE
Our Nominating and Corporate Governance Committee’s responsibilities include:
•
•
•
•
•
•
•
The members of our Nominating and Corporate Governance Committee are Dr. Ausiello and Messrs. BerensonMr. Berenson. Mr. Behar served as a member of the Nominating and Behar.Corporate Governance Committee until October 2023. Mr. Berenson serves as the Chairperson of the Nominating and Corporate Governance Committee.
The Nominating and Corporate Governance Committee met three times in 2021.2023.
27
SCIENCE AND CLINICAL DEVELOPMENT COMMITTEE
ExecutiveOur Science and Director CompensationClinical Development Committee’s responsibilities include:
As
The members of our Science and Clinical Development Committee are Drs. Ausiello, Dere and Fraser. Dr. Ausiello serves as the Chairperson of the Science and Clinical Development Committee.
The Science and Clinical Development Committee met two times in 2023.
28
Executive Compensation
Compensation Discussion and Analysis
As a smaller reporting company, we are eligible to follow the reduced disclosure obligations regarding executive compensation disclosure is intended to comply with the requirements applicablethat apply to smaller reporting companies. Although the SEC rules allow the CompanyHowever, in this section, we have voluntarily elected to provide less detail about itsinclude executive compensation program,disclosure under certain sections of Item 402 of Regulation S-K that are not required of a smaller reporting company.
GENERAL
In the Compensation Discussion and Talent Committee is committed to providing the information necessary to help stockholders understand its executive compensation-related decisions in order to make voting determinations regarding our executive compensation program. Accordingly, this section discusses the material componentsAnalysis (“CD&A”) set forth below, we provide an overview and analysis of the executive compensation program offeredawarded to or earned by our named executive officers or(“NEOs”) identified in the Summary Compensation Table below during fiscal 2023, including the elements of our compensation program for NEOs, identified below.material compensation decisions made under that program for fiscal 2023 and the material factors considered in making those decisions. For 2021,the year ended December 31, 2023, our NEOs, which consisted of our principal executive officer and our two most other highly compensated executive officers for fiscal year 2023, were:
•
David Arkowitz,•
Matthew Henn, Ph.D.•
Stockholder Advisory Vote on Executive Compensation
At our 2023 Annual Meeting of Stockholders, our stockholders voted in a non-binding, advisory vote to approve the compensation of our NEOs. The Compensation and Talent Committee reviewed the result of this vote, and, in light of the approval by a substantial majority of our stockholders of the compensation programs described in our 2023 proxy statement (representing approximately 99.4% of the votes cast), did not implement any significant changes to our executive compensation program as a result of the vote.
EXECUTIVE SUMMARY
Compensation Highlights:
Details of Our Compensation Program
Compensation Philosophy
Our executive compensation program has been designed to motivate, reward, attract and retain high caliber management deemed essential to ensure our success. The program seeks to align executive compensation with our short- and long-term objectives, business strategy and financial performance.
We intend that the total compensation of our NEOs reflect a “pay for performance” compensation philosophy. As detailed in the next section, we generally target total compensation relative to the 50th percentile of our peer group, but the Compensation and Talent Committee retains discretion to adjust compensation reflecting individual factors or performance. Our executive compensation program consists of three primary components: base salary, annual performance-based cash incentive awards and periodic equity-based incentives, which, in 2021,2023, consisted of grants of stock options, restricted stock units, and for Mr. Arkowitz,performance-based restricted stock units.
29
Compensation Setting Process
Role of Board of Directors, Compensation and Talent Committee and Executive Officers
The Compensation and Talent Committee evaluates and recommends the compensation for our President and Chief Executive Officer to the Board and makes compensation decisions regarding all of our other NEOs. Our President and Chief Executive Officer reviews the performance of our other executive officers and then makes recommendations to the Compensation and Talent Committee to assist it in determining their compensation levels. While the Compensation and Talent Committee utilizes this information and values management’s observations with regard to compensation, the ultimate decisions regarding executive compensation are made by the Compensation and Talent Committee, except the compensation for our President and Chief Executive Officer, which is made by the Board.
Role of Compensation Consultant
Our Compensation and Talent Committee has the authority under its charter to engage the services of a consulting firm or other outside advisor to assist it in designing our compensation programs and in making executive compensation decisions. Our Compensation and Talent Committee has engaged Aon’s Human Capital Solutions practice, a division of Aon plc (“Aon”), as itsengages compensation consultantconsultants to assess and make recommendations with respect to the amount and types of compensation to provide our executives and directors. Aon plc, or Aon, advised our Compensation and Talent Committee through September 2022, and Alpine Rewards, LLC, or Alpine, has advised our Compensation and Talent Committee since October 2022. When making executive compensation decisions in 2021,2023, our Compensation and Talent Committee considered advice and data provided by Aon and also met with Aon to discuss compensation of our executive officers, including Mr. Shaff. Aon reported directly to the Compensation and Talent Committee; however, Mr. Shaff, our President and Chief Executive Officer, consulted with Aon with respect to his assessments of the compensation of executive officers other than himself.Alpine. Aon provided the Compensation and Talent Committee with 2023 peer group information, and market information thatAlpine reviewed this data with the Compensation and Talent Committee used when determiningthe Compensation and Talent Committee determined whether our
executive compensation is competitive, commensurate with the executive officers’ responsibilities and consistent with market trends in executive compensation practices for comparable companies. AonOur Compensation and Talent Committee also providesmet with Alpine to discuss compensation of our executive officers, including our President and Chief Executive Officer. Alpine reported directly to the Compensation and Talent Committee; however, our President and Chief Executive Officer consulted with Alpine with respect to his assessments of the compensation of executive officers other than himself.
Alpine provided no additional services to us that are unrelated to executiveother than in its role of advising our Compensation and director compensation. The fees for these additional services were less than $120,000 during 2021.Talent Committee. The Compensation and Talent Committee has considered the adviser independence factors required under SEC rules and Nasdaq listing standards as they relate to AonAlpine and does not believe Aon’sAlpine's work in 20212023 raised a conflict of interest.
The Compensation and Talent Committee recognizes the very competitive market for executive talent in our industry, and the importance of attracting and retaining strong talent as our business continues to evolve. Our positioning on compensation is intended to keep the Company competitive while strongly incentivizing performance and appropriately controlling executive compensation cost.
2023 Peer Group
In connection with the Compensation and Talent Committee’s review of our executive compensation programs in late 2020,2022, Aon provided market data and analysis relative to a peer group selected by the Compensation and Talent Committee in consultation with Aon. Peer group companies were selected based on the following parameters:
|
|
|
|
|
Compared to the prior year, the criteria used to review and select peers was adjusted as follows: the company typestage of development was updated to exclude companies in turnaround situations; the stage of development changed from companies with product candidates in Phase 2 or 3 clinical trials to companies with product candidates in Phase 3 clinical trials orinclude only companies that have recently submitted a NDA or BLA; the market capitalization range was broadeneddecreased (as the range was previously $100$200 million to $600 million)$2 billion); and the headcount range was broadenedincreased (as the range was previously limited100 to companies with less than 3501,000 employees); and the revenue was increased (as the range was previously under $250 million).
Given these changes in peer company profiles, Aon recommended and, in August 2020,September 2022, the Compensation and Talent Committee approved, the removal of sevennine companies (two of which were acquired) and the addition of eight new peer companies that better aligned with the Company’s profile to form the following compensation peer group:
Albireo Pharma Inc.* | Amylyx Pharmaceuticals, Inc.* | Arcutis Biotherapeutics, Inc.* | ||
Ardelyx, Inc. | Atara Biotherapeutics Inc.* | Atea Pharmaceuticals, Inc.* |
30
bluebird bio Inc.* | Chinook Therapeutics, Inc.* | Crinetics Pharmaceuticals, Inc.* | ||
Deciphera Pharmaceuticals, Inc. | Evelo Biosciences Inc. | ImmunoGen, Inc. | ||
Inovio Pharmaceuticals, Inc. | ||||
Karyopham Therapeutics, Inc. | MacroGenics, Inc. | |||
Revance Therapeutics, Inc. | ||||
Rhythm Pharmaceuticals, Inc. | ||||
* Indicates new peer companies. |
The Compensation and Talent Committee believes the constituent companies in the peer group are similar to us based on development stage, revenue, industry, executive role considerations and market capitalization and are representative of our competitors for talent and capital. Aon providesAlpine provided the Compensation and Talent Committee with a competitive assessment of our compensation program for executive officers against the peer group with respect to pay philosophies, pay mix, cash and equity-linked compensation. The Compensation and Talent Committee utilizes the peer group in making compensation decisions to help ensure our compensation program for executive officers adheres to
our compensation philosophy of maintaining executive pay that rewards performance while remaining competitive, commensurate with the executive officers’ responsibilities and consistent with market trends in executive compensation practices for comparable companies.
2021 SUMMARY COMPENSATION TABLE
Elements of Our Executive Compensation Program
Historically, and for fiscal 2023, our executive compensation program consisted of the following elements, each established as part of our program in order to achieve the compensation objective specified below:
Name and Principal Position | Year | Salary ($) | Bonus | Stock Awards ($)(1) | Option Awards | Non-Equity Incentive Plan Compensation ($)(2) | All Other Compensation ($)(3) | Total ($) | ||||||||||||||||||||||||
Eric D. Shaff | 2021 | 625,400 | — | — | 8,758,582 | 319,000 | 8,698 | 9,711,680 | ||||||||||||||||||||||||
President and Chief Executive Officer | 2020 | 590,000 | — | — | 2,050,600 | 486,750 | 8,550 | 3,135,900 | ||||||||||||||||||||||||
David Arkowitz | 2021 | 265,417 | 50,000 | (4) | 916,100 | 3,896,775 | 93,994 | 4,409 | 5,226,695 | |||||||||||||||||||||||
Executive Vice President, Chief Financial Officer and Head of Business Development | ||||||||||||||||||||||||||||||||
Matthew Henn, Ph.D. | 2021 | 430,000 | — | — | 2,960,014 | 153,080 | 8,698 | 3,551,792 | ||||||||||||||||||||||||
Executive Vice President and Chief Scientific Officer | ||||||||||||||||||||||||||||||||
Compensation Element |
|
Base Salary | Attracts and retains talented executives, recognizes individual roles and responsibilities, and provides stable income. |
Cash-Based Incentive Compensation | Promotes short-term performance objectives and rewards executives for their contributions toward achieving those objectives. |
Equity-Based Compensation | Aligns executives’ interests with our stockholders’ interests, emphasizes long-term financial and operational performance, and helps retain executive talent. |
Severance and Other Benefits Potentially Payable upon Termination of | Aids in attracting and |
Retirement, Health, Welfare and Additional Benefits | Aids in |
|
|
|
NARRATIVE DISCLOSURE TO 2021 SUMMARY COMPENSATION TABLE
The primaryWe do not currently have, and we do not expect to have, formal policies relating to the allocation of total compensation among the various elements of our compensation forprogram.
Base Salary
The base salaries of our NEOs are base salary, annual cash bonusesan important part of their total compensation package, and long- term, equity-based compensation awards. Our NEOs also participate in employee benefit plansare intended to reflect their respective positions, duties and programs that we offer to our other full-time employees on the same basis and have from time to time received relocation or other expense reimbursements from us.
Base Salary
responsibilities. Our NEOs receive base salary to compensate them for the satisfactory performance of duties to our company.Company. The base salary payable to each NEO is intended to provide a visible and stable fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities. Base salaries provide our NEOs with a reasonable degree of financial certainty and stability.
Our Compensation and Talent Committee periodically reviews NEO base salaries in consultation with management and Aonthe committee's compensation consultant to determine whether any adjustments are necessary or appropriate. We elected to increase the 2024 base salary of Dr. Young by 8.7% to better align her salary with the 50th percentile of our peer group while considering individual performance, experience, and level of contribution. Our other NEOs did not receive salary increases in 2024. The following table shows the annual base salaries of our NEOs for 2020, 20212022, 2023 and 2022. Except as otherwise noted, all2024. All annual base salary increases were effective January 1 of the given year.
Name | 2020 Annual Base Salary ($) | 2021 Annual Base Salary ($) | 2022 Annual Base Salary ($) |
| 2022 Annual Base |
|
| 2023 Annual Base |
|
| 2024 Annual Base |
| ||||||||||||
Eric D. Shaff | 590,000 | 625,400 | 661,600 |
|
| 661,600 |
|
|
| 685,000 |
|
|
| 685,000 |
| |||||||||
David Arkowitz(1) | — | 455,000 | 470,900 | |||||||||||||||||||||
Matthew Henn, Ph.D. | 397,000 | 430,000 | 450,000 | |||||||||||||||||||||
Teresa L. Young, Ph.D. |
|
| 405,000 |
|
|
| 437,000 |
|
|
| 475,000 |
| ||||||||||||
Lisa von Moltke, M.D. |
|
| 490,000 |
|
|
| 515,000 |
|
|
| 515,000 |
| ||||||||||||
|
Annual Cash Bonuses
Cash-Based Incentive Compensation
Our NEOs generally have the opportunity to earn annual performance bonuses based on the achievement of short-term performance goals. The NEOs’ 20212023 target annual cash bonuses, expressed as a percentage of base salary, were: 60% for Mr. Shaff and 40% for Mr. Arkowitz and Dr. Henn.the other NEOs.
31
Our Compensation and Talent Committee generally determines annual bonuses for our NEOs by multiplying (a) base salary, by (b) target cash bonus percentage, by (c) the level of achievement of corporate and/or individual performance objectives. In addition, the Compensation and Talent Committee retains discretion to adjust annual bonuses as it determines to be appropriate to reflect company performance, individual performance or other factors that the committee believes to be appropriate. For our NEOs, except Mr. Arkowitz and Dr. Henn,Shaff, the achievement of 20212023 corporate objectives was weighted 80% and the achievement of individual objectives was weighted at 20%. Mr. Shaff’s 20212023 bonus was weighted 100% on corporate objectives.
The table below provides additional details about the Compensation and Talent Committee’s assessment of our actual performance against our 20212023 corporate objectives:objectives. Individual objectives are analyzed based on the business judgement of the Compensation and Talent Committee's subjective analysis of individual goals achieved in support of each of our corporate goals and are not based on specified performance measures.
2021 Corporate Objective | Weighting | Assessed Performance | ||||||
Program Objectives(1) |
| |||||||
SER-109: BLA submission readiness | 22 | % | 19.8 | % | ||||
SER-287: Phase 2b topline results available | 15 | % | 7.5 | % | ||||
SER-301: Evaluate phase 1b cohort 1 results | 10 | % | 8.5 | % | ||||
SER-155: Initiate phase 1b study | 4 | % | 3.4 | % | ||||
Franchise Objectives(2) |
| |||||||
IBD franchise strategy development | 5 | % | 4 | % | ||||
CDI franchise strategy development | 5 | % | 5 | % | ||||
Functional Objectives(3) |
| |||||||
Business development planning | 6 | % | 6.3 | % | ||||
CMC-QS | 6 | % | 6.3 | % | ||||
Commercial | 5 | % | 4.6 | % | ||||
Finance | 15 | % | 13.1 | % | ||||
Human Resources | 5 | % | 6.3 | % | ||||
Compliance | 2 | % | 2 | % | ||||
Total | 100 | % | 86.8 | % | ||||
2023 Corporate Objectives | Weighting |
| Assessed |
| ||
VOWST |
| |||||
Approval: Secure FDA approval of VOWST at Prescription Drug User Fee Act, or PDUFA |
| 25 | % |
| 25 | % |
Supply Responsiveness: Deliver VOWST inventory targets in budget; improve responsiveness to VOWST market demand; continue to progress capacity expansion projects |
| 10 | % |
| 10 | % |
Launch: Deliver new patient starts |
| 9 | % |
| 14 | % |
LCM: Establish VOWST recurrent Clostridioides difficile infection lifecycle management plan |
| 13 | % |
| 13 | % |
Pipeline, Platform, and Technology |
|
|
|
| ||
SER-155: Deliver Phase 1b Cohort 1 Day 100 data; progress Phase 1b Cohort 2 enrollment |
| 19 | % |
| 17 | % |
Business Development: Support pipeline strategy |
| 6 | % |
| 4 | % |
Corporate |
|
|
|
| ||
Finance: Execute financing strategy to strengthen balance sheet |
| 6 | % |
| 4 | % |
Communications: Establish best-in-class IR and PR function |
| 6 | % |
| 3 | % |
People & Culture: Drive an engaged and committed culture focused on achieving our strategic and operational milestones |
| 6 | % |
| 8 | % |
Total |
| 100 | % |
| 98 | % |
|
|
|
In February 2022,2024, the Compensation and Talent Committee determined our 2023 corporate objectives were achieved at the 98% level. However, in consultation with management and based on guidance from Aon,Alpine, the Compensation and Talent Committee determinedelected to award Messrs. Shaff and Arkowitz and Dr. Henn 2021 performance bonuses of 85% of the corporate objective portion of their targetDr. Young’s and Dr. von Moltke's 2023 bonus levels,based on 90% corporate achievement and Mr. Arkowitz received 102.5%Shaff's 2023 bonus based on 80% corporate achievement. These adjustments were made primarily to recognize our share price performance for the year and Dr. Henn received 105% ofin keeping with our pay for performance compensation philosophy. For Drs. Young and von Moltke, the individual objectivesperformance portion of their target bonus level. Mr. Arkowitz’s 2021 bonus was pro-ratedachieved at 120% for his partial year of employment.Dr. Young and 130% for Dr. von Moltke. The actual amounts paid to our NEOs under our 20212023 annual cash bonus program are set forth in the 20212023 Summary Compensation Table above.below.
Our Compensation and Talent Committee periodically reviews NEO target bonus amounts in consultation with management and Aonthe committee's compensation consultant to determine whether any adjustments are necessary or appropriate. The NEOs’ 20222024 target annual cash bonus opportunity, expressed as a percentage of base salary, is: 60% for Mr. Shaff 40% for Mr. Arkowitz and 40% for Dr. Henn.our other NEOs, which percentages are unchanged from 2023.
Equity-Based Compensation
In 2021,We maintain the long-term2015 Incentive Award Plan (as amended and restated, the “2015 Plan”), under which we may grant equity incentive component of the compensationawards to directors, employees and consultants of our NEOs consistedCompany and our affiliates, to enable us to attract and retain services, skills and experience of stock options and,these individuals, which we believe are essential to our long-term success. We do not currently have any formal policy for Mr. Arkowitz, restricted stock units. determining the number or types of equity-based awards to grant to NEOs.
We typically grant equity incentive awards to
employees when they commence employment with us and annually in conjunction with our review of individual performance. We may grant additional awards in the discretion of our Board of Directors or Compensation and Talent Committee. Our stock options allow employees to purchase shares of our Common Stock at a price equal to the fair market value of our Common Stock on the grant date, as determined under the applicable equity incentive plan, and may be intended to qualify as “incentive stock options” under the Internal Revenue Code of 1986, as amended (the “Code”). We believe stock options directly align the interests of our NEOs with those of our shareholders while ensuring the retention of our executive team. Our restricted stock units represent the right to receive one share of our Common Stock or its cash value equivalent upon vesting. In order to remain competitive in the market, we granted both restricted stock units and performance-based restricted stock units to our NEOs for the first time in 2023 in connection with their annual award. We also awarded Dr. Young additional time-based restricted stock units in 2023 for retention purposes.
32
Our stock options typically vest as to 25% of the shares subject to the option on the first anniversary of the grant date (or service commencement date for initial grants) and in 12 quarterly installments during the three-year period thereafter, subject to the holder’s continued service with us. Our restricted stock units typically vest as to 25% of the restricted stock units on the first 15th day of the calendar month that immediately follows the first anniversary of the grant date, and as to 6.25% of the restricted stock units upon completion of each three consecutive months of service during the three-year period thereafter, subject to the holder’s continued service with us. From time to time, our Board of Directors or Compensation and Talent Committee may also construct alternate vesting schedules as it determines are appropriate to motivate particular employees. Stock options and restricted stock units granted to our employees may be subject to accelerated vesting in certain circumstances, as described in the section titled “Employment Agreements.“Employment Agreements.”
In the first quarter of each calendar year, we generally awardgrant equity incentive awards based on competitive market practices and the retention value that NEOs have from past equity awards.
In February 2023, we granted stock options, based on the achievement of the previous year’s performance goals. We awarded time-based restricted stock optionsunits and performance-based restricted stock units to our NEOs during 2021 in the following amounts with exercise prices equal to the fair market value of our common stock on the date of grant and with the vesting schedule indicated in the table below.
| ||||
| ||||
| ||||
| ||||
Name |
| Options |
|
| Time-Based Restricted Stock Units Granted (#) |
| Performance-Based Restricted Stock Units Granted (#)(4) |
| ||
Eric D. Shaff |
|
| 250,000 |
|
| 125,000(2) |
|
| 55,675 |
|
Teresa L. Young, Ph.D. |
|
| 85,000 |
|
| 127,500(3) |
|
| 22,721 |
|
Lisa von Moltke, M.D. |
|
| 95,000 |
|
| 47,500(2) |
|
| 27,489 |
|
(1) Option vests as to 25% of the total shares subject to the option on the first anniversary of the vesting commencement date, and as to 6.25% of the shares subject to the option upon each consecutive three months of service during the three-year period thereafter, subject to the holder’s continued employment with us through the applicable vesting date and potential accelerated vesting as described in the section titled “Employment Agreements.” (2) Restricted stock unit award vests as to 25% |
In addition, we awarded Mr. Shaff and Dr. Henn stock options during 2021 covering 143,000 shares and 36,750 shares, respectively, that were eligible to vest on the date that both of the following conditions are satisfied: (i) our Board of Directors or Compensation and Talent Committee (the “Administrator”) determines that the BLA for SER-287 has been approved by the U.S. Food and Drug Administration (“FDA”) and (ii) the Administrator determines that the primary endpoint in the SER-287 Phase 2b study has been attained on or prior to September 30, 2021 (the “Performance Condition”), provided that if the Administrator determines that the Performance Condition was not satisfied in the Company’s Phase 2b clinical trial, such stock option would immediately expire and be forfeited for no consideration. Each of these performance-based options expired in 2021 pursuant to their terms.
Additionally, in connection with the commencement of his employment in June 2021, Mr. Arkowitz was granted an award of 5,000 restricted stock units, which are scheduled to vest on June 15, 2022, subject to his continued employment. Mr. Arkowitz was also granted 85,000 restricted stock units in November 2021 that vest (i) as to 50% of the restricted stock units on the first 15th day of the calendar month that immediately follows the first anniversary of the grant date, and as to 6.25% of the restricted stock units upon completion of each three consecutive months of service during the three-year period thereafter, subject to the holder’s continued employment with us through the applicable vesting date and potential accelerated vesting as described in the section titled “Employment Agreements.”
Severance and Change in Control Arrangements
We are party to employment agreements with each of our NEOs, which generally provide for severance benefits and payments upon a termination without cause or for good reason and enhanced severance benefits and payments if such termination is within 60 days prior to or 12 months following a change in control. Our Compensation and Talent Committee believes that these types of arrangements are necessary to attract and retain executive talent and are a customary component of executive compensation. Particularly, such arrangements can mitigate a potential disincentive for our NEOs when they are evaluating a potential acquisition of the Company and can encourage retention through the conclusion of the transaction. The payments and benefits provided under our severance and change in control arrangements are designed to be competitive with market practices. A description of these arrangements, as well as information on the dateestimated payments and benefits that the Administrator determines that the BLA for SER-109 hasour NEOs would have been approved by the FDA (the “BLA Approval”) on or prioreligible to receive as of December 31, 2023.2023, are set forth in “Employment Agreements and —Potential Payments Upon Termination or Change in Control” below.
Retirement, Health, Welfare and Additional Benefits
Our NEOs are eligible to participate in our employee benefit plans and programs, including medical and dental benefits, life insurance, short-term disability and long-term disability to the same extent as our other full-time employees, subject to the terms and eligibility requirements of those plans. We also
We also sponsor a 401(k) defined contribution plan in which our NEOs may participate, subject to limits imposed by the Code, to the same extent as all of our other full-time employees. During 2021,2023, we made discretionary employer matching contributions equal to 50% of elective contributions made by participants in the 401(k) plan, up to 6% of a participant’s eligible compensation. These matching contributions are fully vested as of the date on which the contribution is made. We believe that providing a vehicle for tax-deferred retirement savings through our 401(k) plan adds to the overall desirability
33
of our employee compensation package and further incentivizes our employees, including our NEOs, in accordance with our compensation policies. We did not provide perquisites or special personal benefits to our NEOs for 2021.2023.
Tax Considerations
As a general matter, the Compensation and Talent Committee reviews and considers the various tax and accounting implications of the compensation programs we utilize.
34
Executive Compensation Tables
2023 Summary Compensation Table
Name and Principal Position |
| Year |
| Salary |
|
| Stock |
|
| Option |
|
| Non-Equity |
|
| All Other |
|
| Total ($) |
| ||||||
Eric D. Shaff |
| 2023 |
|
| 685,000 |
|
|
| 993,713 |
|
|
| 1,140,925 |
|
|
| 328,800 |
|
|
| 9,898 |
|
|
| 3,158,336 |
|
President and Chief Executive Officer |
| 2022 |
|
| 661,600 |
|
|
| — |
|
|
| 2,938,500 |
|
|
| 397,000 |
|
|
| 9,148 |
|
|
| 4,006,248 |
|
Teresa L. Young, Ph.D. |
| 2023 |
|
| 437,000 |
|
|
| 826,216 |
|
|
| 387,915 |
|
|
| 167,808 |
|
|
| 1,519 |
|
|
| 1,820,458 |
|
Executive Vice President, Chief Commercial and Strategy Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Lisa von Moltke, M.D. |
| 2023 |
|
| 515,000 |
|
|
| 412,440 |
|
|
| 433,552 |
|
|
| 201,880 |
|
|
| 8,269 |
|
|
| 1,571,141 |
|
Executive Vice President and Chief Medical Officer |
| 2022 |
|
| 490,000 |
|
|
| — |
|
|
| 1,028,475 |
|
|
| 208,000 |
|
|
| 8,139 |
|
|
| 1,734,614 |
|
Grants of Plan-Based Awards in Fiscal 2023
The following table provides supplemental information relating to grants of plan-based awards made during fiscal 2023 to help explain information provided above in our Summary Compensation Table. All awards are granted under the 2015 Plan. This table presents information regarding all grants of plan-based awards occurring during fiscal 2023.
Name | Grant Date | Estimated Future Payout Under Non-Equity Incentive Plan Awards Target ($)(1) |
| Estimated Future Payout Under Equity Incentive Plan Awards Target (#)(2) |
| All Other Stock | All Other Option Awards: Number of Securities Underlying Options (#)(3) |
| Exercise or Base Price of Option Awards ($/Sh) |
| Grant Date Fair Value of Stock and Option Awards(4) |
| |||||
Eric Shaff | 2/3/2023 |
|
|
|
|
|
| 250,000 |
|
| 5.50 |
|
| 1,140,925 |
| ||
| 2/3/2023 |
|
|
| 55,675 |
|
|
|
|
|
|
| 306,213 |
| |||
| 2/3/2023 |
|
|
|
| 125,000(5) |
|
|
|
|
| 687,500 |
| ||||
|
|
| 411,000 |
|
|
|
|
|
|
|
|
|
| ||||
Teresa L. Young, Ph.D. | 2/3/2023 |
|
|
|
|
|
| 85,000 |
|
| 5.50 |
|
| 387,915 |
| ||
| 2/3/2023 |
|
|
| 22,721 |
|
|
|
|
|
|
| 124,966 |
| |||
| 2/3/2023 |
|
|
|
| 42,500(5) |
|
|
|
|
| 233,750 |
| ||||
| 2/3/2023 |
|
|
|
| 85,000(6) |
|
|
|
|
| 467,500 |
| ||||
|
|
| 174,800 |
|
|
|
|
|
|
|
|
|
| ||||
Lisa von Moltke, M.D. | 2/3/2023 |
|
|
|
|
|
| 95,000 |
|
| 5.50 |
|
| 433,552 |
| ||
| 2/3/2023 |
|
|
| 27,489 |
|
|
|
|
|
|
| 151,190 |
| |||
| 2/3/2023 |
|
|
|
| 47,500(5) |
|
|
|
|
| 261,250 |
| ||||
|
|
| 206,000 |
|
|
|
|
|
|
|
|
|
| ||||
Outstanding Equity Awards at 2023 Fiscal Year End
|
|
|
| Option Awards |
| Stock Awards |
| |||||||||||||||||
Name |
| Grant |
| Number of |
|
| Number of |
|
| Option |
|
| Option |
| Number of Shares or Units of Stock That Have Not Vested |
|
| Market Value of Shares or Units of Stock That Have Not Vested |
| |||||
Eric D. Shaff |
| 2/3/2023 (1) |
|
|
|
|
| 250,000 |
|
|
| 5.50 |
|
| 2/2/2033 |
|
|
|
|
|
| |||
|
| 2/3/2023 (3) |
|
|
|
|
|
|
|
|
|
|
|
|
| 125,000 |
|
|
| 175,000 |
| |||
|
| 2/3/2023 (4) |
|
|
|
|
|
|
|
|
|
|
|
|
| 27,837 |
|
|
| 38,972 |
| |||
|
| 2/4/2022 (1) |
|
| 218,750 |
|
|
| 281,250 |
|
|
| 7.38 |
|
| 2/3/2032 |
|
|
|
|
|
| ||
|
| 2/4/2021 (1) |
|
| 240,625 |
|
|
| 109,375 |
|
|
| 26.34 |
|
| 2/3/2031 |
|
|
|
|
|
| ||
|
| 1/29/2020 (1) |
|
| 937,500 |
|
|
| 62,500 |
|
|
| 3.30 |
|
| 1/28/2030 |
|
|
|
|
|
| ||
|
| 1/25/2019 (1) |
|
| 275,000 |
|
|
|
|
|
| 6.01 |
|
| 1/24/2029 |
|
|
|
|
|
| |||
|
| 1/30/2018 (1) |
|
| 120,000 |
|
|
|
|
|
| 10.42 |
|
| 1/29/2028 |
|
|
|
|
|
| |||
|
| 1/26/2017 (1) |
|
| 105,000 |
|
|
|
|
|
| 9.89 |
|
| 1/25/2027 |
|
|
|
|
|
| |||
|
| 2/1/2016 (1) |
|
| 78,500 |
|
|
|
|
|
| 26.20 |
|
| 1/31/2026 |
|
|
|
|
|
| |||
|
| 6/26/2015 (2) |
|
| 100,000 |
|
|
|
|
|
| 18.00 |
|
| 6/25/2025 |
|
|
|
|
|
| |||
|
| 12/9/2014 (2) |
|
| 233,454 |
|
|
|
|
|
| 7.79 |
|
| 12/8/2024 |
|
|
|
|
|
| |||
Teresa L. Young, Ph.D. |
| 2/3/2023 (1) |
|
|
|
|
| 85,000 |
|
|
| 5.50 |
|
| 2/2/2033 |
|
|
|
|
|
| |||
|
| 2/3/2023 (3) |
|
|
|
|
|
|
|
|
|
|
|
|
| 42,500 |
|
|
| 59,500 |
| |||
|
| 2/3/2023 (4) |
|
|
|
|
|
|
|
|
|
|
|
|
| 11,360 |
|
|
| 15,904 |
| |||
|
| 2/3/2023 (5) |
|
|
|
|
|
|
|
|
|
|
|
|
| 85,000 |
|
|
| 119,000 |
| |||
|
| 2/4/2022 (1) |
|
| 52,500 |
|
|
| 67,500 |
|
|
| 7.38 |
|
| 2/3/2032 |
|
|
|
|
|
| ||
|
| 2/4/2021 (1) |
|
| 61,875 |
|
|
| 28,125 |
|
|
| 26.34 |
|
| 2/3/2031 |
|
|
|
|
|
| ||
|
| 6/29/2020 (1) |
|
| 262,500 |
|
|
| 37,500 |
|
|
| 4.67 |
|
| 6/28/2030 |
|
|
|
|
|
| ||
Lisa von Moltke, M.D. |
| 2/3/2023 (1) |
|
|
|
|
| 95,000 |
|
|
| 5.50 |
|
| 2/2/2033 |
|
|
|
|
|
| |||
|
| 2/3/2023 (3) |
|
|
|
|
|
|
|
|
|
|
|
|
| 47,500 |
|
|
| 66,500 |
| |||
|
| 2/3/2023 (4) |
|
|
|
|
|
|
|
|
|
|
|
|
| 13,744 |
|
|
| 19,242 |
| |||
|
| 2/4/2022 (1) |
|
| 76,562 |
|
|
| 98,438 |
|
|
| 7.38 |
|
| 2/3/2032 |
|
|
|
|
|
| ||
|
| 2/4/2021 (1) |
|
| 75,625 |
|
|
| 34,375 |
|
|
| 26.34 |
|
| 2/3/2031 |
|
|
|
|
|
| ||
|
| 4/6/2020 (1) |
|
| 284,375 |
|
|
| 40,625 |
|
|
| 3.32 |
|
| 4/5/2030 |
|
|
|
|
|
| ||
Option Exercises and Stock Vested in Fiscal 2023
The following table shows the stock vested for our NEOs during fiscal 2023. None of our NEOs exercised stock options during fiscal 2023.
| Option Awards | Stock Awards | Stock Awards |
| |||||||||||||||||||||||||||||||||||||||||||
Name | Vesting Commencement Date | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Underlying | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Number of | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | Number of Shares Acquired |
| Value Realized |
| ||||||||||||||||||||||||||||||||
Eric D. Shaff | 2/4/2021 | (1) | — | 350,000 | — | 26.34 | 2/3/2031 |
| 27,838 |
| 38,416 |
| ||||||||||||||||||||||||||||||||||
| 1/29/2020 | (1) | 437,500 | 562,500 | — | 3.30 | 1/28/2030 | ||||||||||||||||||||||||||||||||||||||||
| 1/25/2019 | (1) | 189,062 | 85,938 | — | 6.01 | 1/24/2029 | ||||||||||||||||||||||||||||||||||||||||
| 1/30/2018 | (1) | 112,500 | 7,500 | — | 10.42 | 1/29/2028 | ||||||||||||||||||||||||||||||||||||||||
| 1/26/2017 | (1) | 105,000 | — | — | 9.89 | 1/25/2027 | ||||||||||||||||||||||||||||||||||||||||
| 2/1/2016 | (1) | 78,500 | — | — | 26.20 | 1/31/2026 | ||||||||||||||||||||||||||||||||||||||||
| 6/26/2015 | (2) | 100,000 | — | — | 18.00 | 6/25/2025 | ||||||||||||||||||||||||||||||||||||||||
| 12/9/2014 | (2) | 233,454 | — | — | 7.79 | 12/8/2024 | ||||||||||||||||||||||||||||||||||||||||
David Arkowitz | 6/1/2021 | (1) | — | 270,000 | — | 20.19 | 5/31/2031 | |||||||||||||||||||||||||||||||||||||||
| 6/15/2022 | (3) | 5,000 | 41,650 | |||||||||||||||||||||||||||||||||||||||||||
| N/A | 85,000 | (4) | 708,050 | |||||||||||||||||||||||||||||||||||||||||||
Matthew Henn | 2/4/2021 | (1) | — | 130,000 | — | 26.34 | 2/3/2031 | |||||||||||||||||||||||||||||||||||||||
| 1/29/2020 | (1) | 131,250 | 168,750 | — | 3.30 | 1/28/2030 | ||||||||||||||||||||||||||||||||||||||||
| 1/25/2019 | (1) | 22,969 | 22,969 | — | 6.01 | 1/24/2029 | ||||||||||||||||||||||||||||||||||||||||
| 1/30/2018 | (1) | 56,250 | 3,750 | — | 10.42 | 1/29/2028 | ||||||||||||||||||||||||||||||||||||||||
| 6/19/2017 | (5) | — | — | 70,000 | 10.84 | 6/18/2027 | ||||||||||||||||||||||||||||||||||||||||
| 1/26/2017 | (1) | 3,750 | — | — | 9.89 | 1/25/2027 | ||||||||||||||||||||||||||||||||||||||||
| 2/1/2016 | (1) | 12,500 | — | — | 26.20 | 1/31/2026 | ||||||||||||||||||||||||||||||||||||||||
| 6/26/2015 | (2) | 40,000 | — | 18.00 | 6/25/2025 | |||||||||||||||||||||||||||||||||||||||||
Teresa L. Young, Ph.D. |
| 11,361 |
| 15,678 |
| |||||||||||||||||||||||||||||||||||||||||
Lisa von Moltke, M.D. |
| 13,745 |
| 18,968 |
| |||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
We have entered into employment agreements with each of our NEOs. Certain key terms of these agreements are described below.
36
Eric D. Shaff
Under the terms of our employment agreement with Mr. Shaff, (which we last amended in January 2021), if we terminate Mr. Shaff’s employment without cause or he resigns for good reason, each within the meaning of his employment agreement, he will be entitled to receive 18 months of continued
base salary, up to 18 months of continued medical, dental or vision coverage pursuant to COBRA, if elected, and immediate vesting of any time-based equity awards that would have otherwise become vested solely as a result of Mr. Shaff’s continued service during the twelve month period following his termination of employment. Mr. Shaff’s employment agreement provides that, upon any such termination within 60 days prior to or 12 months following a change in control, in lieu of the benefits described in the previous sentence, Mr. Shaff is entitled to accelerated vesting of his time-based equity awards, 18 months of continued base salary, up to 18 months of continued medical, dental or vision coverage pursuant to COBRA, if elected, and a lump sum cash amount equal to 1.5 times his target bonus for the year of termination.
Mr. Shaff has also agreed to refrain from disclosing our confidential information during or at any time following his employment with us and from competing with us or soliciting our employees or consultants for 12 months following termination of his employment.
David Arkowitz
InMr. Shaff’s employment agreement contains a parachute payment “best pay” provision, under which payments and benefits in connection with the commencement of his employmenta change in 2021, we entered into ancontrol made pursuant to the employment agreement withor otherwise will either be made to Mr. Arkowitz. The employment agreement provides for, among other things, a sign-on bonusShaff in full or as to such lesser amount as which would result in no portion of $50,000, payable in a single lump sum shortly following the commencement of his employment. This sign-on bonus ispayments and benefits being subject to repayment if Mr. Arkowtiz resigns his employment other than for good reason or we terminate his employment for cause, each withinan excise tax under Section 4999 of the meaningCode, whichever of his employment agreement, prior to the anniversary of his employment start date.foregoing amounts is greater on an after-tax basis.
Teresa L. Young, Ph.D. and Lisa von Moltke, M.D.
Under the terms of our employment agreement with Mr. Arkowitz,each respective executive, if we terminate Mr. Arkowitzthe executive other than for cause or hethe executive resigns for good reason, Mr. Arkowitzeach within the meaning of the respective employment agreement, the executive will be entitled to receive 12 months of continued base salary and up to 12 months of continued medical, dental or vision coverage pursuant to COBRA, if elected. Mr. Arkowitz’sEach executive's employment agreement provides that, upon any such termination within 60 days prior to or 12 months following a change in control, in lieu of the benefits described in the previous sentence, Mr. Arkowitzthe executive is entitled to accelerated vesting of histhe executive's time-based equity awards, 12 months of continued base salary, up to 12 months of continued medical, dental or vision coverage pursuant to COBRA, if elected, and a lump sum cash amount equal to 1.0 times histhe executive's target bonus for the year of termination.
For purposes
Each of Mr. Arkowitz’sthe employment agreements contains a parachute payment “best pay” provision, under which payments and benefits in connection with a change in control made pursuant to the employment agreement “cause”or otherwise will either be made to the NEO in full or as to such lesser amount as which would result in no portion of the payments and “good reason” havebenefits being subject to an excise tax under Section 4999 of the same meanings as in Mr. Shaff’s employment agreement. Mr. ArkowitzCode, whichever of the foregoing amounts is greater on an after-tax basis.
Each executive has also agreed to refrain from disclosing our confidential information during or at any time following histhe executive's employment with us and from competing with us or soliciting our employees or consultants for 12 months following termination of histhe executive's employment.
Matthew Henn, Ph.D.
UnderPotential Payments Upon Termination or Change in Control
In accordance with SEC rules, the termsfollowing table summarizes the payments that would be made to certain of our NEOs upon the occurrence of certain qualifying terminations of employment, agreementassuming such NEO’s termination of employment with Dr. Henn (which we last amended in January 2021), if we terminate Dr. Henn other than for cause or he resigns for good reason, each within the meaning of his employment agreement, Dr. Henn will be entitled to receive 12 months of continued base salaryCompany occurred on December 31, 2023 and, up to 12 months of continued medical, dental or vision coverage pursuant to COBRA, if elected. Dr. Henn’s employment agreement provideswhere relevant, that upon any such termination within 60 days prior to or 12 months following a change in control in lieu of the benefits describedCompany occurred on December 31, 2023. Amounts shown in the previous sentence, Dr. Henntable below do not include (1) accrued but unpaid salary and (2) other benefits earned or accrued by the NEOs during employment that are available to all salaried employees, such as accrued vacation.
37
Name | Benefit | Termination Without Cause or for Good Reason (no Change in Control) ($) |
| Termination Without Cause or for Good Reason in Connection with a Change in Control) ($)(1) |
| ||
Eric Shaff | Continued Base Salary |
| 1,027,500 |
|
| 1,027,500 |
|
| Bonus Lump Sum |
| 0 |
|
| 493,200 |
|
| Continued Healthcare |
| 54,417 |
|
| 54,417 |
|
| Equity Acceleration (2) |
| 115,534 |
|
| 213,972 |
|
| Total (3) |
| 1,197,450 |
|
| 1,789,089 |
|
Teresa L. Young, Ph.D. | Continued Base Salary |
| 437,000 |
|
| 437,000 |
|
| Bonus Lump Sum |
| 0 |
|
| 167,808 |
|
| Continued Healthcare |
| 12,453 |
|
| 12,453 |
|
| Equity Acceleration (2) |
| 0 |
|
| 194,404 |
|
| Total (3) |
| 449,453 |
|
| 811,665 |
|
Lisa von Moltke, M.D. | Continued Base Salary |
| 515,000 |
|
| 515,000 |
|
| Bonus Lump Sum |
| 0 |
|
| 201,880 |
|
| Continued Healthcare |
| 13,116 |
|
| 13,116 |
|
| Equity Acceleration (2) |
| 0 |
|
| 85,742 |
|
| Total (3) |
| 528,116 |
|
| 815,737 |
|
Pay Versus Performance
In accordance with the SEC’s disclosure requirements regarding pay versus performance, this section presents the SEC-defined “Compensation Actually Paid,” or CAP. Also required by the SEC, this section compares CAP to various measures used to gauge our performance. CAP is entitleda supplemental measure to accelerated vestingbe viewed alongside performance measures as an addition to the philosophy and strategy of his time-basedcompensation-setting discussed elsewhere in this proxy statement, not in replacement.
Pay Versus Performance Table
The following table sets forth information concerning the compensation of our NEOs for each of the fiscal years ended December 31, 2021, 2022 and 2023, and our financial performance for each such fiscal year:
Year | Summary Compensation Table Total for PEO ($) |
| Compensation Actually Paid to PEO ($)(1)(2) |
| Average Summary Compensation Table Total for Non-PEO NEOs ($) |
| Average Compensation Actually Paid to Non-PEO NEOs ($)(1)(2) |
| Value of Initial Fixed $100 Investment Based on Total Shareholder Return ($) | Net Income ($) |
| |||||
2023 |
| 3,158,336 |
|
| (517,565 | ) |
| 1,695,800 |
|
| 260,831 |
| 41 |
| (119,548,000 | ) |
2022 |
| 4,006,248 |
|
| 1,574,321 |
|
| 1,706,633 |
|
| 1,109,334 |
| 162 |
| (250,157,000 | ) |
2021 |
| 9,711,680 |
|
| (10,683,446 | ) |
| 4,389,244 |
|
| (448,135 | ) | 241 |
| (65,578,000 | ) |
Year | PEO | Non-PEO NEOs | |
2023 | Eric Shaff | Teresa L. Young, Ph.D. and Lisa von Moltke, M.D. | |
2022 | Eric Shaff | David Arkowitz, Lisa von Moltke, M.D., Matthew Henn, Ph.D., and Paula A. Cloghessy | |
2021 | Eric Shaff | David Arkowitz and Matthew Henn, Ph.D. |
Compensation actually paid to our NEOs represents the “Total” compensation reported in the Summary Compensation Table for the applicable fiscal year, as adjusted as follows:
38
Adjustments | 2023 |
| ||||
| PEO |
| Average Non-PEO NEOs |
| ||
Deduction for Amounts Reported under the “Stock Awards” and “Option Awards” Columns in the Summary Compensation Table for Applicable FY |
| (2,134,638 | ) |
| (1,030,062 | ) |
Increase Based on ASC 718 Fair Value of Awards Granted during Applicable FY that Remain Unvested as of Applicable FY End, Determined as of Applicable FY End |
| 508,247 |
|
| 246,012 |
|
Increase Based on ASC 718 Fair Value of Awards Granted during Applicable FY that Vested during Applicable FY, Determined as of Vesting Date |
| 38,416 |
|
| 17,323 |
|
Increase/deduction for Awards Granted during Prior FY that were Outstanding and Unvested as of Applicable FY End, Determined Based on Change in ASC 718 Fair Value from Prior FY End to Applicable FY End |
| (1,456,981 | ) |
| (498,826 | ) |
Increase/deduction for Awards Granted during Prior FY that Vested During Applicable FY, Determined Based on Change in ASC 718 Fair Value from Prior FY End to Vesting Date |
| (630,945 | ) |
| (169,416 | ) |
Deduction of ASC 718 Fair Value of Awards Granted during Prior FY that were Forfeited during Applicable FY, Determined as of Prior FY End |
| 0 |
|
| 0 |
|
Increase Based on Dividends or Other Earnings Paid during Applicable FY prior to Vesting Date |
| 0 |
|
| 0 |
|
Increase Based on Incremental Fair Value of Options/SARs Modified during Applicable FY |
| 0 |
|
| 0 |
|
Deduction for Change in the Actuarial Present Values Reported under the “Change in Pension Value and Nonqualified Deferred Compensation Earnings” Column of the Summary Compensation Table for Applicable FY |
| 0 |
|
| 0 |
|
Increase for Service Cost and, if Applicable, Prior Service Cost for Pension Plans |
| 0 |
|
| 0 |
|
TOTAL ADJUSTMENTS |
| (3,675,901 | ) |
| (1,434,969 | ) |
Narrative Disclosure to Pay Versus Performance Table
Relationship Between Financial Performance Measures
The graphs below compares the compensation actually paid to our PEO and the average of termination.the compensation actually paid to our remaining NEOs, with (i) our cumulative TSR, and (ii) our net income, in each case, for the fiscal years ended December 31, 2021, 2022 and 2023. TSR amounts reported in the graph assume an initial fixed investment of $100.
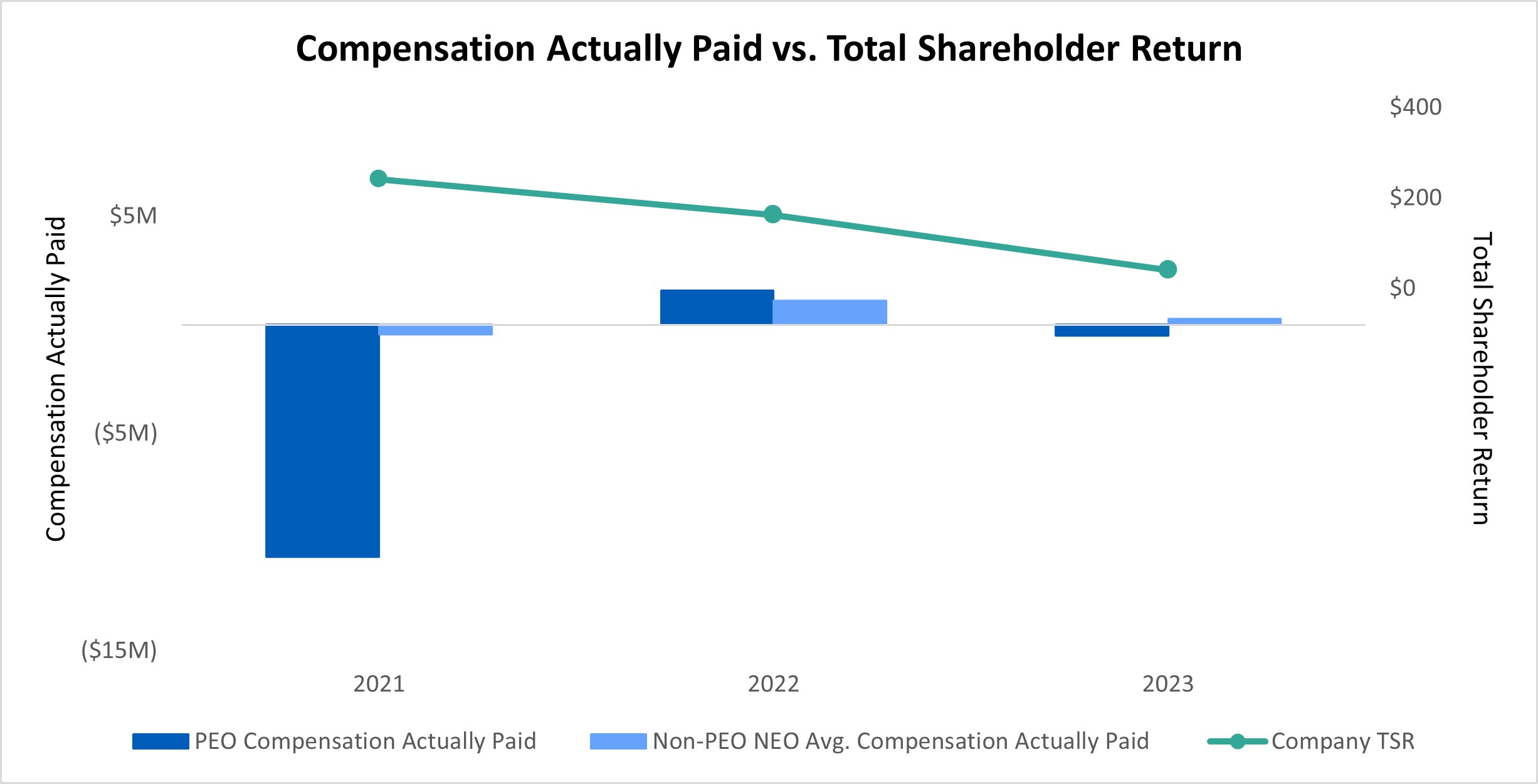
39
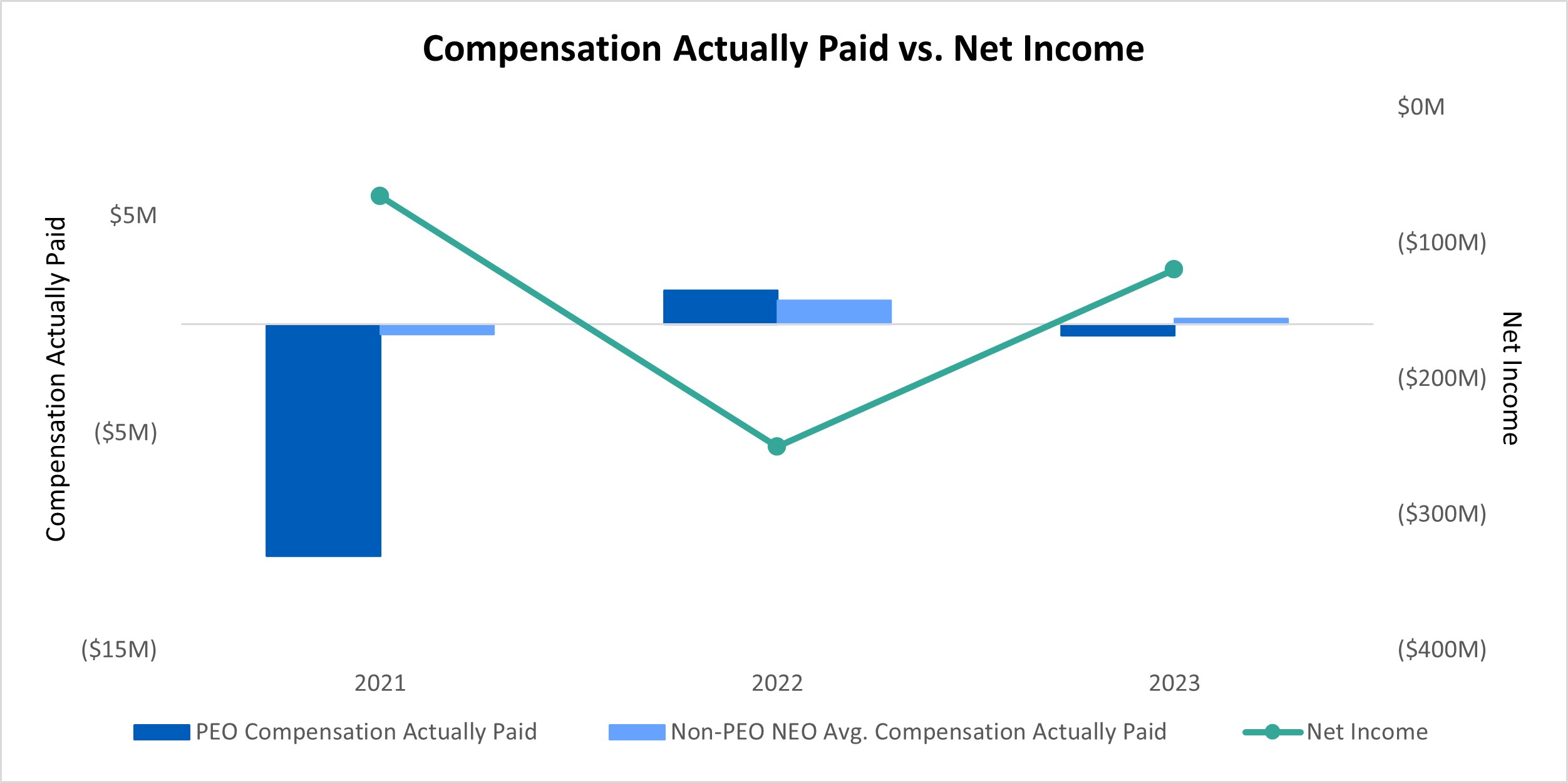
For purposes of Dr. Henn’s employment agreement, “cause” and “good reason” have the same meanings as in Mr. Shaff’s employment agreement. Dr. Henn has also agreed to refrain from disclosing our confidential information during or at any time following his employment with us and from competing with us or soliciting our employees or consultants for 12 months following termination of his employment.
40
DIRECTOR COMPENSATIONDirector Compensation
Directors who are also our employees do not receive compensation for their service on our Board. Mr. Shaff served as a director and executive officer of our company during 2021.2023. Refer to the 20212023 Summary Compensation Table and related narrative disclosure above for information regarding the compensation Mr. Shaff received from us during 2021.2023.
We maintain a compensation program for our non-employee directors providing for each non-employee director to receive the following amounts for serving on our Board:
•
•
•
•
chairman•
•
chairman•
•
chairman•
•
The amounts set forth above reflect changes that were made to this program in June 2021 to decrease the number of shares subject to the Initial Award from 60,000 to 35,000 and the number of shares subject to the Subsequent Award from 30,000 to 23,000 and to increase the cash compensation for serving on our Board and on a committee of our Board.
Stock options granted to our non-employee directors under the program have an exercise price equal to the fair market value of our Common Stock on the grant date. The stock options granted upon a director’s initial election or appointment vest in four annual installments following the grant date. The stock options granted annually to directors vest in a single installment on the earlier of the day before the next annual meeting of stockholders or the first anniversary of the grant date. In addition, all unvested stock options vest in full immediately prior to a change in control.
Each member of our Board is entitled to be reimbursed for reasonable travel and other expenses incurred in connection with attending meetings of the Board and any committee of the Board on which he or she serves.
20212023 DIRECTOR COMPENSATION TABLE
Name |
| Fees Earned or |
|
| Option Awards |
|
| Total ($) |
| |||
Dennis A. Ausiello, M.D. |
|
| 63,042 |
|
|
| 159,226 |
|
|
| 222,268 |
|
Grégory Behar(2) |
|
| 37,500 |
|
|
| 159,226 |
|
|
| 196,726 |
|
Stephen A. Berenson |
|
| 90,000 |
|
|
| 159,226 |
|
|
| 249,226 |
|
Paul R. Biondi |
|
| 52,500 |
|
|
| 159,226 |
|
|
| 211,726 |
|
Willard H. Dere, M.D. |
|
| 66,007 |
|
|
| 159,226 |
|
|
| 225,233 |
|
Claire M. Fraser, Ph.D.(3) |
|
| 61,063 |
|
|
| 377,088 |
|
|
| 438,151 |
|
Kurt C. Graves |
|
| 60,000 |
|
|
| 159,226 |
|
|
| 219,226 |
|
Richard N. Kender |
|
| 73,466 |
|
|
| 159,226 |
|
|
| 232,692 |
|
Meryl S. Zausner(4) |
|
| 32,697 |
|
|
| — |
|
|
| 32,697 |
|
Name | Fees Earned or Paid in Cash ($) | Option Awards ($)(1) | Total ($) | |||||||||
Stephen A. Berenson | 77,154 | 338,006 | 415,160 | |||||||||
Willard H. Dere, M.D. | 51,074 | 338,006 | 389,080 | |||||||||
Richard N. Kender | 65,480 | 338,006 | 403,486 | |||||||||
Grégory Behar | 44,724 | 338,006 | 382,730 | |||||||||
Dennis A. Ausiello, M.D. | 44,724 | 338,006 | 382,730 | |||||||||
Kurt C. Graves | 53,118 | 338,006 | 391,124 | |||||||||
Meryl S. Zausner | 55,618 | 338,006 | 393,624 | |||||||||
Paul R. Biondi | 46,765 | 338,006 | 384,771 | |||||||||
41
Name | Option Awards Outstanding as of | |||
Dennis A. Ausiello, M.D. | 258,000 | |||
Grégory Behar | — | |||
Stephen A. Berenson | 153,000 | |||
Paul R. Biondi | 153,000 | |||
Willard H. Dere, M.D. | 183,000 | |||
Claire M. Fraser, Ph.D. | 83,000 | |||
Kurt C. Graves | 213,000 | |||
Richard N. Kender | 273,000 | |||
| ||||
| ||||
| ||||
Meryl S. Zausner | ||||
| — | |||
EQUITY COMPENSATION PLAN INFORMATION
42
Equity Compensation Plan Information
The following table provides information on our equity compensation plans as of December 31, 2021.2023.
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | Weighted Average Exercise Price of Outstanding Options Warrants and Rights | Number of Securities Available for Future Issuance Under Equity Compensation Plans (excludes securities reflected in first column)(1) |
| Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
|
| Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
|
| Number of Securities Available for Future Issuance Under Equity Compensation Plans (excludes securities reflected in first column)(1) |
|
| ||||||||||||
Equity compensation plans approved by security holders(2) | 12,251,944 | (3) | $ | 11.10 | (4) | 4,530,289 | (5) |
|
| 18,104,800 |
| (3) | $ | 9.66 |
| (4) |
| 4,812,098 |
| (5) | |||||
Equity compensation plans not approved by security holders | — | — | — | ||||||||||||||||||||||
Equity compensation plans not approved by security holders(6) |
|
| 117,116 |
|
| $ | 4.94 |
|
|
| 2,382,884 |
|
| ||||||||||||
Total | 12,251,944 | $ | 11.10 | 4,530,289 |
|
| 18,221,916 |
|
| $ | 9.64 |
|
|
| 7,194,982 |
|
| ||||||||
|
43
|
|
|
|
Security Ownership of Certain Beneficial Owners and Management
Common Stock
COMMON STOCK
The following table sets forth certain information with respect to holdings of our Common Stock by (i) stockholders who beneficially owned more than 5% of the outstanding shares of our Common Stock, and (ii) each of our directors (which includes all nominees), each of our named executive officers and all directors and executive officers as a group as of April 25, 2022,February 12, 2024, unless otherwise indicated. The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC. Under these rules, beneficial ownership includes any shares as to which a person has sole or shared voting power or investment power. Applicable percentage ownership is based on 92,230,428145,557,567 shares of Common Stock outstanding as of April 25, 2022.February 12, 2024. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of Common Stock subject to options, or other rights held by such person that are currently exercisable or will become exercisable within 60 days of April 25, 2022February 12, 2024 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
Unless otherwise indicated, the address of each beneficial owner listed below is 200 Sidney Street,101 Cambridgepark Drive, Cambridge, MA 02139.02140. We believe, based on information provided to us, that each of the stockholders listed below has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
NAME OF BENEFICIAL OWNER | NUMBER | PERCENTAGE | ||||||
5% or Greater Stockholders | ||||||||
Entities affiliated with Flagship Pioneering1 | 14,378,802 | 15.6 | % | |||||
FMR LLC2 | 13,777,070 | 14.9 | % | |||||
Nestlé Health Science US Holdings, Inc.3 | 7,892,890 | 8.6 | % | |||||
Federated Hermes Global Investment Management Corp. 4 | 6,914,136 | 7.5 | % | |||||
BlackRock, Inc.5 | 5,862,691 | 6.4 | % | |||||
Janus Henderson Group plc6 | 5,055,099 | 5.5 | % | |||||
State Street Corporation7 | 4,785,589 | 5.2 | % | |||||
The Vanguard Group8 | 4,676,265 | 5.1 | % | |||||
Named Executive Officers and Directors | ||||||||
Eric D. Shaff9 | 1,565,108 | 1.7 | % | |||||
David Arkowitz10 | 74,500 | * | ||||||
Matthew Henn, Ph.D. 11 | 357,781 | * | ||||||
Dennis A. Ausiello, M.D.12 | 188,000 | * | ||||||
Grégory Behar13 | 98,000 | * | ||||||
Stephen A. Berenson14 | 71,589 | * | ||||||
Paul R. Biondi15 | 68,000 | * | ||||||
Willard H. Dere, M.D.16 | 113,000 | * | ||||||
Kurt C. Graves17 | 143,000 | * | ||||||
Richard N. Kender18 | 203,000 | * | ||||||
Meryl S. Zausner19 | 90,500 | * | ||||||
All executive officers and directors as a group (16 persons)20 | 4,081,807 | 4.2 | % | |||||
NAME OF BENEFICIAL OWNER |
| NUMBER |
|
| PERCENTAGE |
| ||
5% or Greater Stockholders |
|
|
|
|
|
| ||
Entities affiliated with Flagship Pioneering1 |
|
| 23,117,045 |
|
|
| 15.9 | % |
FMR LLC2 |
|
| 19,388,579 |
|
|
| 13.3 | % |
Nestlé S.A.3 |
|
| 7,594,038 |
|
|
| 5.2 | % |
BlackRock, Inc.4 |
|
| 7,339,454 |
|
|
| 5.0 | % |
Named Executive Officers and Directors |
|
|
|
|
|
| ||
Eric D. Shaff5 |
|
| 2,633,824 |
|
|
| 1.8 | % |
Teresa L. Young, Ph.D.6 |
|
| 449,079 |
|
| * |
| |
Lisa von Moltke, M.D.7 |
|
| 540,138 |
|
| * |
| |
Dennis A. Ausiello, M.D.8 |
|
| 223,000 |
|
| * |
| |
Stephen A. Berenson9 |
|
| 121,589 |
|
| * |
| |
Paul R. Biondi10 |
|
| 165,619 |
|
| * |
| |
Willard H. Dere, M.D.11 |
|
| 179,746 |
|
| * |
| |
Claire M. Fraser, Ph.D.12 |
|
| 12,000 |
|
| * |
| |
Kurt C. Graves13 |
|
| 178,000 |
|
| * |
| |
Richard N. Kender14 |
|
| 301,492 |
|
| * |
| |
All executive officers and directors as a group (14 persons)15 |
|
| 7,513,219 |
|
|
| 5.1 | % |
|
|
44
|
|
45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
POLICIES AND PROCEDURES FOR RELATED PERSON TRANSACTIONSPolicies and Procedures for Related Person Transactions
Our Board of Directors has adopted a written Related Person Transaction Policy and Procedures, setting forth the policies and procedures for the review and approval or ratification of related person transactions. Our finance team is primarily responsible for developing and implementing procedures to obtain information regarding potential related person transactions and for determining whether a related person transaction requiring compliance with our policy exists. Our Chief Executive Officer then presents the related person transaction to our Audit Committee. In reviewing and approving any such transaction, our Audit Committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction, the extent of the related person’s interest in the transaction and the conflicts of interest and corporate opportunity provisions under our Code of Business Conduct and Ethics. No director may participate in approval of a related person transaction in which he or she is a related person. Our Audit Committee may also ratify related person transactions that were entered into by management because pre-approval was not feasible and transactions that were not initially recognized as related person transactions. If these transactions are not ratified, our management must make all reasonable efforts to cancel or annul such transactions. Our management must update our Audit Committee on material changes to any approved or ratified related person transaction and provide an annual status report on all then-current related person transactions.
The following are certain transactions, arrangements and relationships with our directors, executive officers and stockholders owning 5% or more of our outstanding Common Stock, or any member of the immediate family of any of the foregoing persons, since January 1, 2020.2023.
TRANSACTIONS WITH RELATED PARTIES
Transactions with Related Parties
In January 2016 we entered into the Collaboration and License Agreement (the “2016 License Agreement”) with Nestec Ltd., which was succeeded in interest by Société des Produits Nestlé S.A. (together with NHSc Pharma Partners, which was succeeded in interest by NHSc Rx License GmbH, “Nestlé”), an affiliate of Nestlé Health Science US Holdings, Inc.S.A., which together with Nestlé, holds approximately 8.6%5.2% of our Common Stock as of April 25, 2022,February 12, 2024, for the development and commercialization of certain of our product candidates in development for the treatment and management of Clostridium difficile infection (“CDI”) and inflammatory bowel disease (“IBD”), including ulcerative colitis and Crohn’s disease. The 2016 License Agreement supports the development of our portfolio of products for CDI and IBD in markets outside of the United States and Canada (the “2016 Licensed Territory”).
Under the 2016 License Agreement, we granted to Nestlé an exclusive, royalty-bearing license to develop and commercialize in the 2016 Licensed Territory certain products based on our microbiome technology that are being developed for the treatment of CDI and IBD, including SER-109,VOWST, SER-262, SER-287 and SER-301 (collectively, the “2016 Collaboration Products”). The 2016 License Agreement sets forth our and Nestlé’s respective obligations for development, commercialization, regulatory and manufacturing and supply activities for the 2016 Collaboration Products with respect to the licensed fields and the 2016 Licensed Territory.
Under the 2016 License Agreement, Nestlé paid us an upfront cash payment of $120.0 million in February 2016 and has agreed to pay us tiered royalties, at percentages ranging from the high single digits to high teens, of net sales of 2016 Collaboration Products in the 2016 Licensed Territory. Additionally, Nestlé has agreed to pay us up to $660.0 million for the achievement of certain development and regulatory milestones and up to an aggregate of $1.125 billion for the achievement of certain commercial milestones related to the sales of 2016 Collaboration Products. We received a $10.0 million milestone payment in 2016 associated with the initiation of our Phase 1b study for
SER-262 in CDI and a $20.0 million milestone payment in 2017 associated with the initiation of our Phase 3 study of SER-109VOWST in multiply recurrent CDI.
In November 2018, we executed the letter agreement with Nestlé (the “Letter Agreement”), modifying certain terms of the 2016 License Agreement. Under the Letter Agreement, Nestlé agreed to accelerate the payment of the $20.0 million Phase 3 commencement milestone to be payable upon the commencement of the Phase 2b study for SER-287. Further, based on the results of the Phase 2b study, the Letter Agreement modifies certain terms and conditions related to the extent and timing of expense reimbursement associated with the ongoing SER-287 clinical trials. The Phase 2b study was initiated and the $40.0 million of milestone payments were received in December 2018. We also received a $10.0 million milestone payment in 2020 associated with the initiation of our Phase 1b study for SER-301. To date, we have received $80.0 million in development milestones under the 2016 License Agreement and the Letter Agreement.
The 2016 License Agreement continues in effect until terminated by either us or Nestlé on the following bases: (i) Nestlé may terminate the 2016 License Agreement in the event of serious safety issues related to any of the 2016 Collaboration
46
Products; (ii) we may terminate the 2016 License Agreement if Nestlé challenges the validity or enforceability of any of our licensed patents; and (iii) either we or Nestlé may terminate the 2016 License Agreement in the event of the other party’s uncured material breach or insolvency. Upon termination of the 2016 License Agreement, all licenses granted to Nestlé by us will terminate, and all rights in and to the 2016 Collaboration Products in the 2016 Licensed Territory will revert to us. If we commit a material breach of the 2016 License Agreement, Nestlé may elect not to terminate the 2016 License Agreement but instead apply specified adjustments to its payment obligations and other terms and conditions of the 2016 License Agreement.
In August 2020, we entered into a Securities Purchase Agreement with Nestlé for the sale of 959,002 shares of our Common Stock at a purchase price of $20.855 per share, for aggregate net proceeds of approximately $19.9 million after deducting offering expenses payable by us (the “concurrent placement”). The concurrent placement was contingent upon the closing of our August 2020 public offering in which we received net proceeds of approximately $243.7 million after deducting underwriting discounts and commissions and offering expenses payable by us.
In July 2021, we entered into a License Agreement (the “2021 License Agreement”) with NHSc Pharma Partners, which was succeeded in interest by NHSc Rx License GmbH (together with Société des Produits Nestlé S.A., their affiliates and their subsidiaries, "Nestlé"). Pursuant to the 2021 License Agreement, we granted to Nestlé, under certain of our patent rights and know how, a co-exclusive, sublicensable (under certain circumstances) license to develop, commercialize and conduct medical affairs activities for (i) therapeutic products based on our microbiome technology (including our SER-109 product candidate)VOWST) that are developed by us or on our behalf for the treatment of CDI and recurrent CDI, as well as any other indications pursued for the products upon mutual agreement of the parties (the “2021 Field”), in the United States and Canada (the “2021 Licensed Territory”), and (ii) our SER-109 product candidateVOWST and any improvements and modifications thereto developed pursuant to the terms of the 2021 License Agreement (the “2021 Collaboration Products”) for any indications in the 2021 Licensed Territory.
The 2021 License Agreement sets forth the parties’ respective obligations for development, regulatory, commercialization, medical affairs, and manufacturing and supply activities for the 2021 Collaboration Products with respect to the 2021 Field and the 2021 Licensed Territory. Pursuant to the 2021 License Agreement, we arewere responsible for, and will useused commercially reasonable efforts in, conducting development of SER-109VOWST in the 2021 Field in the United States until first regulatory approval for SER-109 isVOWST was obtained in the 2021 Field in the United States and in accordance with a development and regulatory activity plan, at our cost, subject to certain exceptions specified in the 2021 License Agreement. We are also responsible for all regulatory affairs related to the 2021 Collaboration Products in the 2021 Field in the 2021 Licensed Territory, at itsour cost, except that expenses incurred for regulatory activities approved by a joint steering committee pursuant to a life cycle management plan for the 2021 Collaboration Products are shared equally between the parties. We will beare now solely responsible for manufacturing and supplying 2021 Collaboration ProductsVOWST for development in the 2021 Field in the 2021 Licensed Territory.
Nestlé has the sole right to commercialize the 2021 Collaboration ProductsVOWST in the 2021 Licensed Territory in accordance with a commercialization plan, subject to our right to elect to provide up to a specified percentage of all promotional details for a certain target audience. Each party will use commercially reasonable efforts to commercialize the 2021 Collaboration ProductsVOWST in the 2021 Licensed Territory in accordance with the commercialization plan. Both parties will perform medical affairs activities for 2021 Collaboration ProductsVOWST in the 2021 Licensed Territory in accordance with a medical affairs plan. We will beare solely responsible for the manufacturing and supply of 2021 Collaboration ProductsVOWST for commercialization under a supply agreement that will be entered intohas been executed between the parties. We will bewere responsible for commercialization and medical affairs activities costs incurred by the parties until first commercial sale of the first 2021 Collaboration Product, or VOWST, in the 2021 Licensed Territory and in accordance with a pre-launch plan, up to a specified cap. FollowingSince the first commercial sale of the first 2021 Collaboration Product,VOWST in June 2023, we will beare entitled to a royaltyshare equally in an amount equal to approximately 50% of theits commercial profits.profits and losses.
In exchange for the grant of the licenses under the 2021 License Agreement, Nestlé agreed to pay us a non-refundable, non-creditable and non-cancellable upfront payment of $175.0 million, which was received in July 2021. Nestlé also agreed to pay us an additional $125.0 million due upon FDA approval of SER-109,VOWST, which was received in May 2023, $10.0 million upon Canadian regulatory approval of SER-109,VOWST, and sales target milestones payments totaling up to $225.0 million.
The 2021 License Agreement continues in effect until all development and commercialization activities for all 2021 Collaboration ProductsVOWST in the 2021 Licensed Territory have permanently ceased. The 2021 License Agreement may be terminated by either party upon sixty days’ written notice for the other party’s material breach that remains uncured during such sixty-day period, or immediately upon written notice for the other party’s insolvency. Nestlé may also terminate the 2021 License Agreement at-will (i) with twelve months’ prior written notice, effective only on or after the third anniversary of first commercial sale of the first 2021 Collaboration ProductVOWST in the 2021 Licensed Territory, (ii) if first commercial sale of the first 2021 Collaboration Product in the 2021 Licensed Territory has not occurred by the fifth anniversary of the effective date of the 2021 License Agreement, with one hundred eighty days’ prior written notice, which must be provided during a specified period set forth in the 2021 License Agreement, or (iii) if regulatory approval for SER-109 is not granted after submission by us of a filing seeking first regulatory approval as set forth in the development and regulatory activity plan, and the parties fail to agree on further development of SER-109 in accordance with the terms of the 2021 License Agreement, with one hundred eighty days’ prior written notice, which must be provided within a specified period set forth in the 2021 License Agreement.Territory. We may also terminate the 2021 License Agreement immediately upon written notice if Nestlé challenges any licensed patent in the 2021 Licensed Territory.
Upon termination of the 2021 License Agreement, all licenses granted to Nestlé by us will terminate. If we commit a material breach of the 2021 License Agreement, Nestlé may elect not to terminate the 2021 License Agreement but instead apply specified adjustments to the payment terms and other terms and conditions of the 2021 License Agreement. The 2021 License Agreement contains customary representations and warranties by the parties, intellectual property
47
provisions including ownership, patent prosecution, enforcement and defense, certain indemnification rights in favor of each party, and customary confidentiality provisions and limitations of liability.
EMPLOYMENT AGREEMENTS
In June 2022, we entered into a securities purchase agreement with Flagship Pioneering Fund VII, L.P. and Nutritional Health LTP Fund, L.P., affiliates of Flagship Pioneering, or Flagship, one of our significant
stockholders, for the sale of 8,738,243 shares of our Common Stock at a purchase price of $3.15 per share as
part of a registered direct offering, which closed on July 5, 2022. We received proceeds from Flagship of $27.5
million.
In July 2022, we entered into a pledge and utilization agreement with Flagship Pioneering Labs TPC, Inc., an affiliate of Flagship, for an option to lease certain manufacturing space. We paid $833 thousand for this option. In June 2023, we elected not to renew the option.
Employment Agreements
We have entered into employment agreements with our executive officers. For more information regarding the employment agreements with our named executive officers, see the section in this proxy statement entitled “Executive and Director Compensation—Employment Agreements.”
INDEMNIFICATION AGREEMENTS
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us or will require us to indemnify each director (and in certain cases their related venture capital funds) and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
48
Compensation and Talent Committee Interlocks and Insider Participation
During the fiscal year ended December 31, 2021,2023, Paul R. Biondi, Kurt C. Graves, and Richard N. Kender and Meryl S. Zausner served as members of our Compensation and Talent Committee. Meryl S. Zausner served as a member of our Compensation and Talent Committee until June 22, 2023. No member of our Compensation and Talent Committee during the fiscal year ended December 31, 20212023 is or has been an officer or employee of our Company. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity that had an executive officer who served as a director on our Board or as a member of our Compensation and Talent Committee during the fiscal year ended December 31, 2021.2023.
49
Stockholders who intend to have a proposal considered for inclusion in our proxy materials for presentation at our 20232025 Annual Meeting of Stockholders pursuant to Rule 14a-8 under the Exchange Act must submit the proposal to our Secretary at our offices at 200 Sidney Street, 4th Floor, Cambridge, MA 02139 in writing not later than December 30, 2022.November 5, 2024.
Stockholders intending to present a proposal at the 20232025 Annual Meeting of Stockholders, but not to include the proposal in our proxy statement, or to nominate a person for election as a director, must comply with the requirements set forth in our Amended and Restated Bylaws. Our Amended and Restated Bylaws require, among other things, that our Secretary receive written notice from the stockholder of record of their intent to present such proposal or nomination not earlier than the close of business on the 120th day and not later than the close of business on the 90th day prior to the anniversary of the preceding year’s annual meeting. Therefore, the Company must receive notice of such a proposal or nomination for the 20232025 Annual Meeting of Stockholders no earlier than the close of business on February 22, 2023December 5, 2024 and no later than the close of business on March 24, 2023.January 4, 2025. The notice must contain the information required by the Amended and Restated Bylaws, a copy of which is available upon request to our Secretary. In the event that the date of the 20232025 Annual Meeting of Stockholders is more than 30 days before or more than 60 days after June 22, 2023,April 4, 2025, then our Secretary must receive such written notice not earlier than the close of business on the 120th day prior to the 20232025 Annual Meeting and not later than the close of business on the 90th day prior to the 20232025 Annual Meeting or, if later, the 10th day following the day on which public disclosure of the date of such meeting is first made by us. SEC rules permit management to vote proxies in its discretion in certain cases if the stockholder does not comply with this deadline and, in certain other cases notwithstanding the stockholder’s compliance with this deadline.
In addition to satisfying the foregoing requirements under our Amended and Restated Bylaws, to comply with the universal proxy rules, (once they become effective), stockholders who intend to solicit proxies in support of director nominees other than our nominees must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act no later than April 24, 2023.Act.
We reserve the right to reject, rule out of order, or take other appropriate action with respect to any proposal that does not comply with these or other applicable requirements.
50
Our Board of Directors is not aware of any matter to be presented for action at the Annual Meeting other than the matters referred to above and does not intend to bring any other matters before the Annual Meeting. However, if other matters should come before the Annual Meeting, it is intended that holders of the proxies named on the Company’s proxy card will vote thereon in their discretion.
51
The accompanying proxy is solicited by and on behalf of our Board of Directors, whose Notice of Annual Meeting is attached to this proxy statement, and the entire cost of such solicitation will be borne by us. In addition to the use of mail, proxies may be solicited by personal interview, telephone, e-mail and facsimile
by our directors, officers and other employees who will not be specially compensated for these services. We will also request that brokers, nominees, custodians and other fiduciaries forward soliciting materials to the beneficial owners of shares held by such brokers, nominees, custodians and other fiduciaries. We will reimburse such persons for their reasonable expenses in connection therewith.
Certain information contained in this proxy statement relating to the occupations and security holdings of our directors and officers is based upon information received from the individual directors and officers.
We intend to file a proxy statement and WHITE proxy card with the SEC in connection with the solicitation of proxies for our 2023 Annual Meeting of Stockholders.
Stockholders may obtain our proxy statement (and any amendments and supplements thereto) and other documents as and when filed by us with the SEC without charge from the SEC’s website at: www.sec.gov.
52
Seres’ Annual Report on Form 10-K
A copy of Seres’ Annual Report on Form 10-K for the fiscal year ended December 31, 2021,2023, including financial statements and schedules thereto, but not including exhibits, as filed with the SEC but not including exhibits, will be (i) mailed with this proxy statement and (ii) sent without charge, along with copies of the exhibits for a reasonable fee, to any stockholder of record on April 25, 2022 without chargeFebruary 12, 2024 upon written request addressed to:
Seres Therapeutics, Inc.
Attention: Secretary
200 Sidney Street101 Cambridgepark Drive
Cambridge, MA 0213902140
A reasonable fee will be charged for copies of exhibits.
You also may access this proxy statement and our Annual Report on Form 10-K at www.proxyvote.com. You also may access our Annual Report on Form 10-K for the year ended December 31, 20212023 at www.serestherapeutics.comwww.serestherapeutics.com.
WHETHER OR NOT YOU PLAN TO ATTEND THE ANNUAL MEETING ONLINE, WE URGE YOU TO VOTE YOUR SHARES VIA THE INTERNET, AS DESCRIBED IN THIS PROXY STATEMENT. IF YOU RECEIVED A COPY OF THE PROXY CARD BY MAIL, YOU MAY SIGN, DATE AND MAIL THE PROXY CARD IN THE ENCLOSED RETURN ENVELOPE. PROMPTLY VOTING YOUR SHARES WILL ENSURE THE PRESENCE OF A QUORUM AT THE ANNUAL MEETING AND WILL SAVE US THE EXPENSE OF FURTHER SOLICITATION.
By Order of the Board of Directors

Thomas J. DesRosier, Secretary
Cambridge, Massachusetts
[ ], 2024
53

SERES THERAPEUTICS LOGO SERES THERAPEUTICS, INC. 101 CAMBRIDGEPARK DRIVE CAMBRIDGE, MA 02140 SCAN TO VIEW MATERIALS & VOTE VOTE BY INTERNET Before The Meeting - Go to www.proxyvote.com or scan the QR Barcode above Use the Internet to transmit your voting instructions and for electronic delivery of information. Vote by 11:59 p.m. Eastern Time on April 29, 2022
|
|
3, 2024. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. During The Meeting - Go to www.virtualshareholdermeeting.com/MCRB2024 You may attend the meeting via the Internet and vote during the meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS:
| V29392-P04490 KEEP |
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
DETACH AND RETURN THIS PORTION ONLY
FOR YOUR RECORDS THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED.
DETACH AND RETURN THIS PORTION ONLY SERES THERAPEUTICS, INC. The Board of Directors recommends you vote FOR the following: For All Withhold All For All Except To withhold authority to vote for any individual nominee(s), mark "For All Except" and write the number(s) of the nominee(s) on the line below. 1. Election of Directors Nominees: 01) Paul R. Biondi 02) Kurt C. Graves The Board of Directors recommends you vote FOR proposals 2 through 5. For Against Abstain 2. Ratify the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024.3. Approval, on an advisory (non-binding) basis, of the compensation of our named executive officers. 4. Approval of an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of Common Stock from 240,000,000 to 360,000,000. 5. Approval of an adjournment of the Annual Meeting, if necessary, to solicit additional proxies if there are not sufficient votes at the time of the Annual Meeting to approve Proposal 4. The Board of Directors recommends you vote AGAINST proposal 6. For Against Abstain 6. A stockholder proposal on Simple Majority Vote, if properly presented. NOTE: To transact such other business as may properly come before the Annual Meeting or any continuation, postponement, or adjournment thereof. Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. Signature [PLEASE SIGN WITHIN BOX] Date Signature (Joint Owners) Date
|
|
|
|
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||||||

Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting:
The Notice and Proxy Statement and Annual Report are available at www.proxyvote.com
| ||||||||||||
V29393-P04490 SERES THERAPEUTICS, INC. Annual Meeting of Stockholders April 4, 2024 8:00 AM EDT This proxy is solicited by the Board of Directors The undersigned stockholder(s) hereby appoint(s) Eric D. Shaff and Thomas J. DesRosier, or either of them, as proxies, each with the power to appoint his substitute, and hereby authorize(s) them to represent and to vote, as designated on the reverse side of this proxy card, all of the shares of common stock of Seres Therapeutics, Inc. that the undersigned stockholder(s) would be entitled to vote if present at the Annual Meeting of Stockholders to be held live via webcast at www.virtualshareholdermeeting.com/MCRB2024 on April 4, 2024 at 8:00 AM EDT, and any adjournment, continuation, or postponement thereof. Such proxies are authorized to vote in their discretion (x) for the election of any person to the Board of Directors if any nominee named herein becomes unable to serve or for good cause will not serve, (y) on any matter that the Board of Directors did not know would be presented at the Annual Meeting by a reasonable time before the proxy solicitation was made, and (z) on such other business as may properly be brought before the meeting or any adjournment, continuation, or postponement thereof. This proxy, when properly executed, will be voted in the manner directed herein by the undersigned stockholder(s). If no such direction is made, this proxy will be voted in accordance with the Board of Directors' recommendations. Continued and to be signed on reverse side
| ||||||
| ||||||
| ||||||

